Abstract
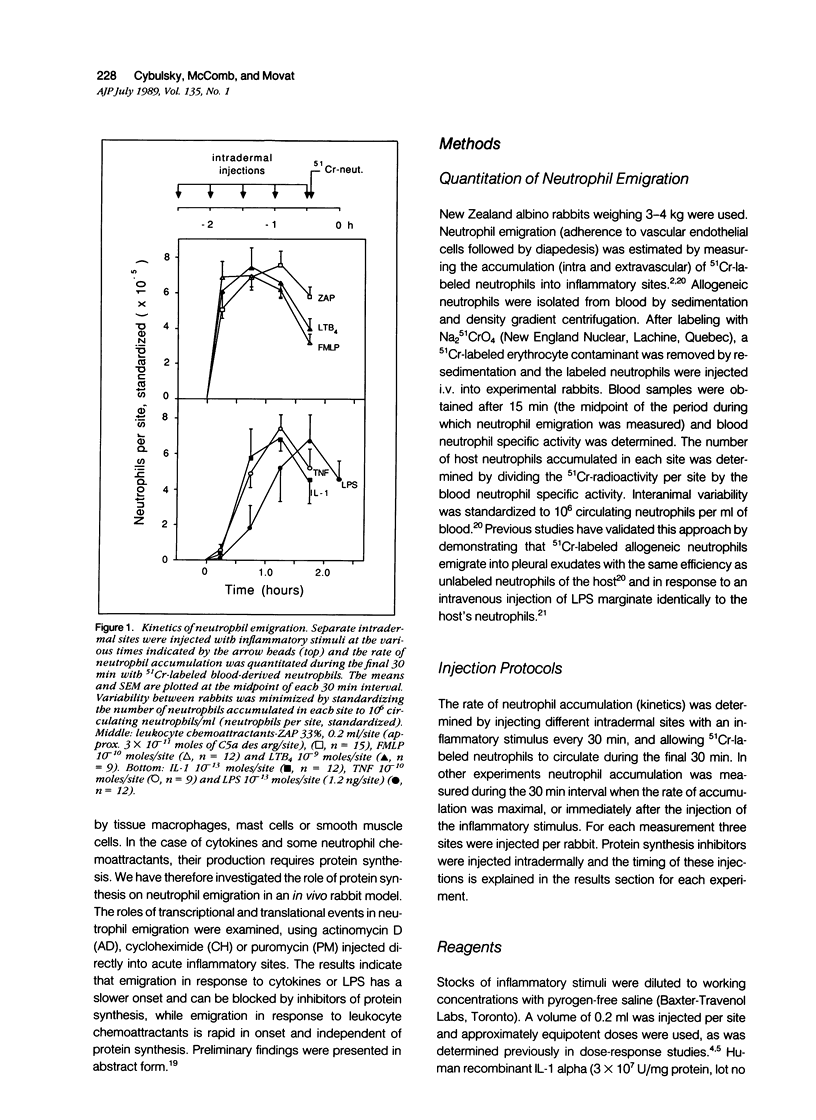
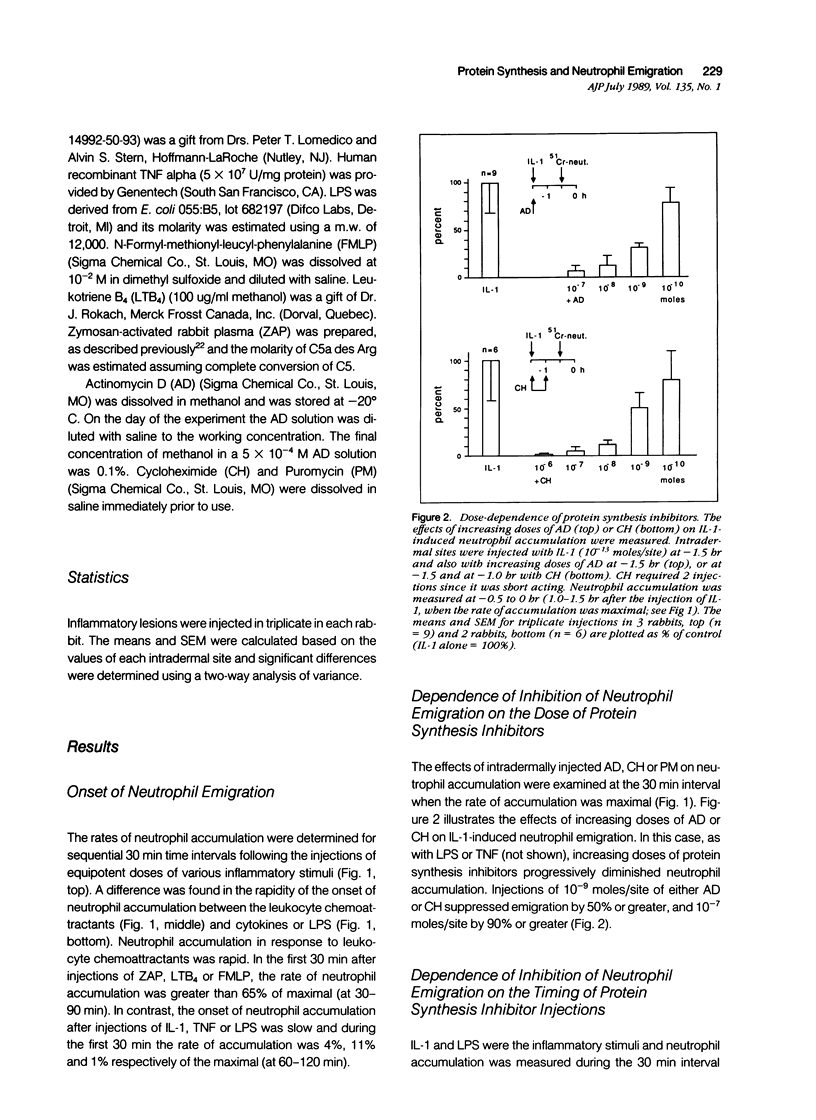
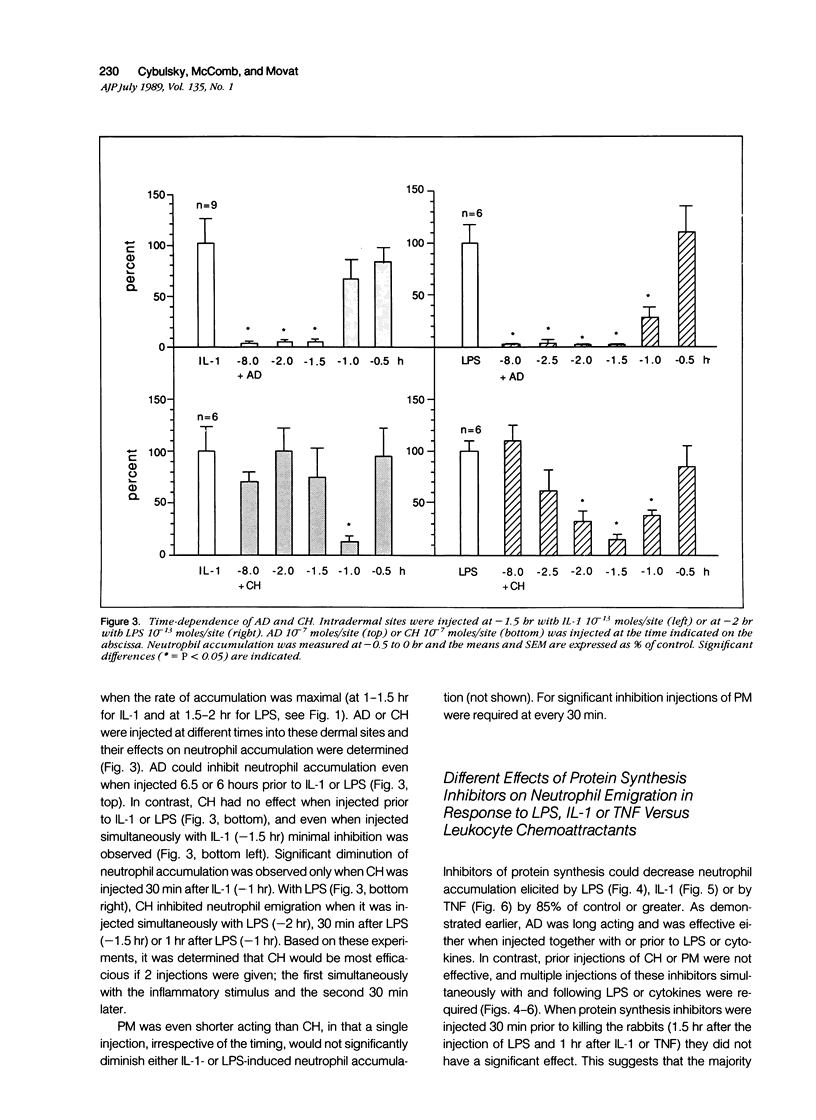
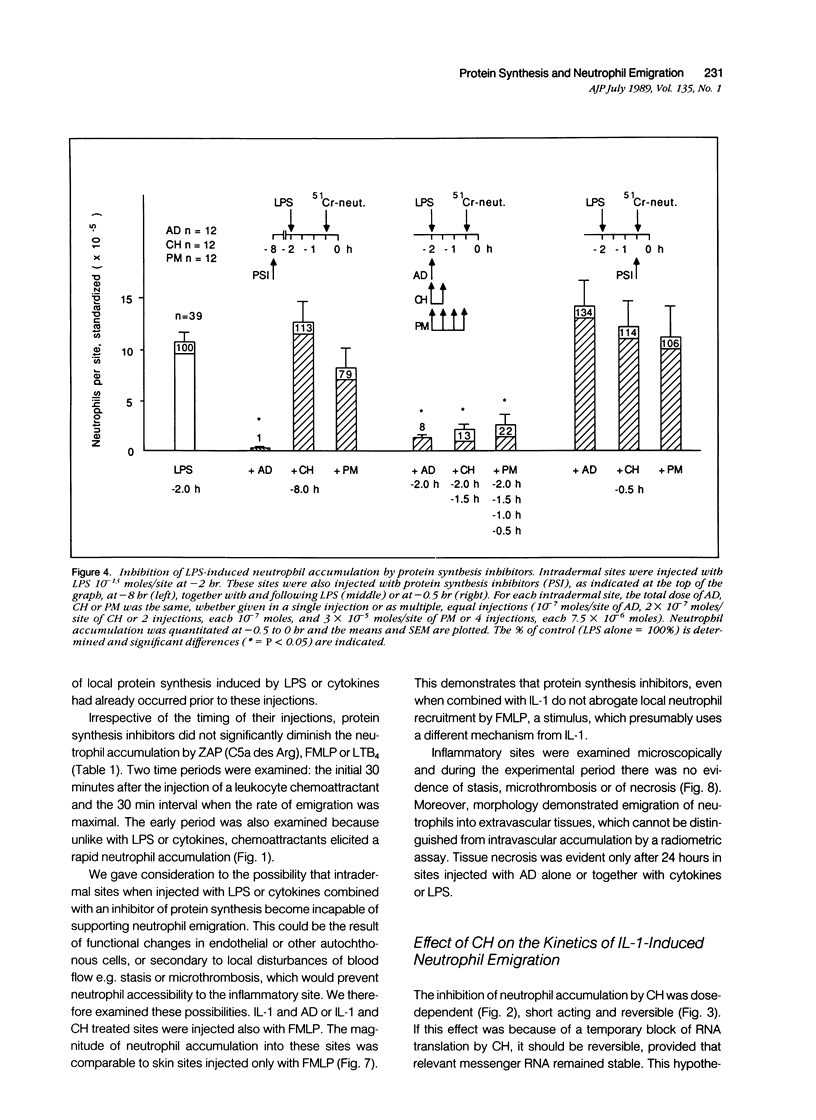
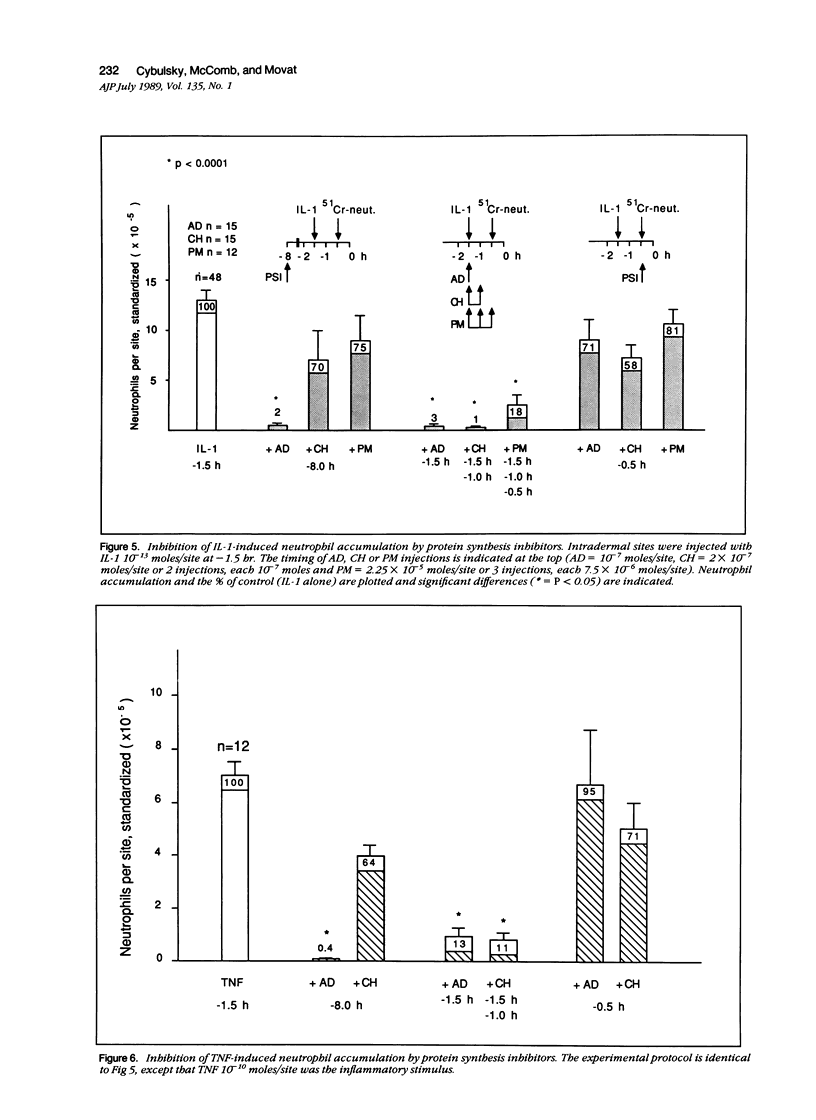
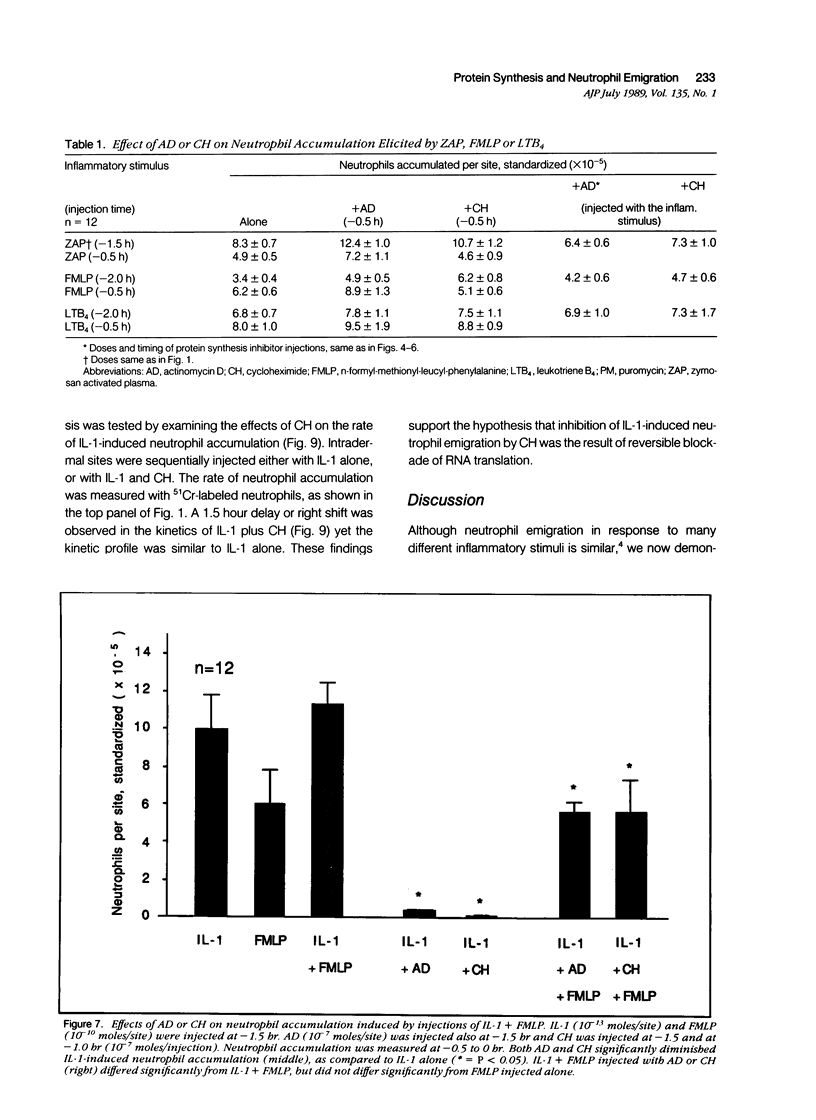
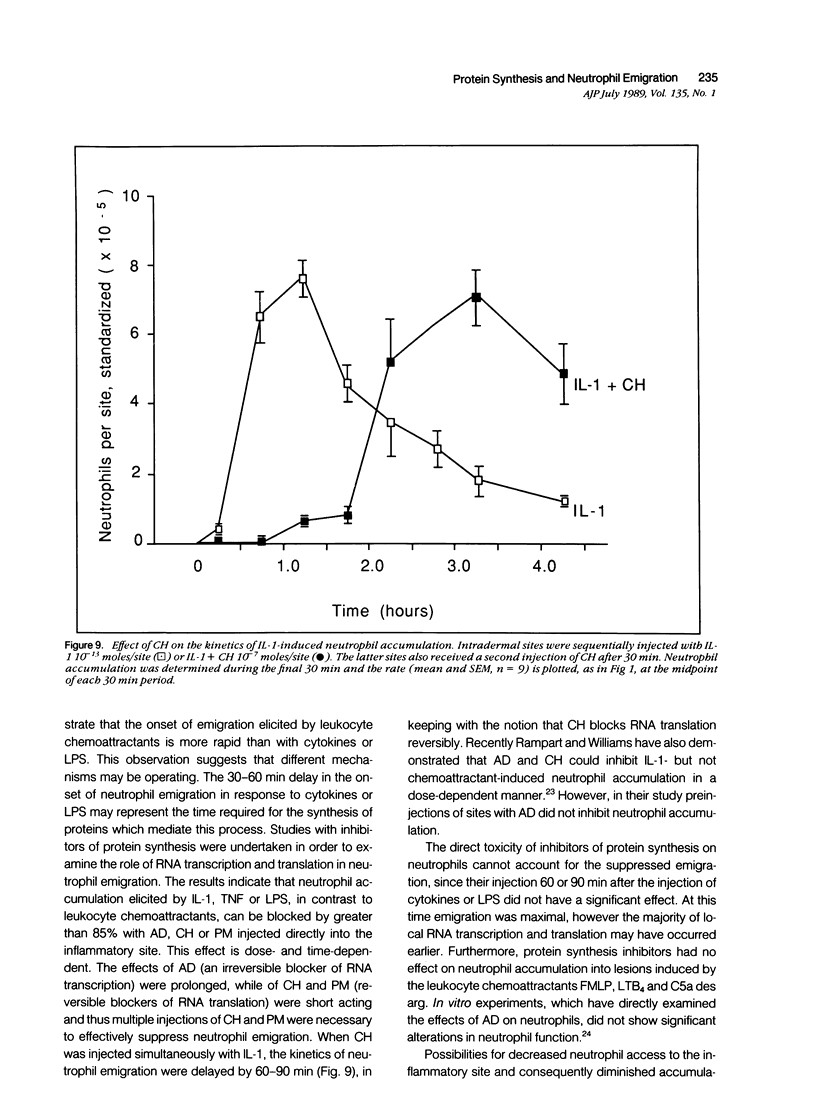
Inflammation constitutes the body's principal mode of defense against infection and other harmful agents. Neutrophil leukocytes are the primary effector cells in this process. The role of protein synthesis in neutrophil emigration into acute inflammatory lesions was examined. Local intradermal injections of actinomycin D, cycloheximide or puromycin could inhibit in a dose- and time-dependent manner neutrophil emigration induced by interleukin-1, tumor necrosis factor alpha or endotoxin, but not by the leukocyte chemoattractants C5a des arg (zymosan-activated plasma), n-formyl-methionyl-leucyl-phenylalanine or leukotriene B4. Maximal inhibition, measured at the time of peak emigration, was greater than 90%. The onset of neutrophil emigration induced by the cytokines or by endotoxin was delayed by 30 to 60 minutes in comparison to the leukocyte chemoattractants. These results demonstrate at least two mechanisms of neutrophil emigration: one with a slower onset and dependence on local RNA transcription and translation and the other rapid in onset and independent of protein synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Bernheim H. A., Dinarello C. A. Effect of protein synthesis inhibitors on leukocytic pyrogen-induced in vitro hypothalamic prostaglandin production. Yale J Biol Med. 1985 Mar-Apr;58(2):179–187. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M. S., Mallett C., VandeVen C., Kempert P., Bennetts G. A., Katz J. Impaired in vitro polymorphonuclear function secondary to the chemotherapeutic effects of vincristine, adriamycin, cyclophosphamide, and actinomycin D. J Clin Oncol. 1986 May;4(5):798–804. doi: 10.1200/JCO.1986.4.5.798. [DOI] [PubMed] [Google Scholar]
- Colditz I. G. Kinetics of tachyphylaxis to mediators of acute inflammation. Immunology. 1985 May;55(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Chemotactic factor-specific desensitization of skin to infiltration by polymorphonuclear leukocytes. Immunol Lett. 1984;8(2):83–87. doi: 10.1016/0165-2478(84)90055-5. [DOI] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Desensitization of acute inflammatory lesions to chemotaxins and endotoxin. J Immunol. 1984 Oct;133(4):2163–2168. [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Colditz I. G., Movat H. Z. The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol. 1986 Sep;124(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Cybulsky I. J., Movat H. Z. Neutropenic responses to intradermal injections of Escherichia coli. Effects on the kinetics of polymorphonuclear leukocyte emigration. Am J Pathol. 1986 Jul;124(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., McComb D. J., Movat H. Z. Neutrophil leukocyte emigration induced by endotoxin. Mediator roles of interleukin 1 and tumor necrosis factor alpha 1. J Immunol. 1988 May 1;140(9):3144–3149. [PubMed] [Google Scholar]
- Cybulsky M. I., Movat H. Z. Experimental bacterial pneumonia in rabbits: polymorphonuclear leukocyte margination and sequestration in rabbit lungs and quantitation and kinetics of 51Cr-labeled polymorphonuclear leukocytes in E. coli-induced lung lesions. Exp Lung Res. 1982 Dec;4(1):47–66. doi: 10.3109/01902148209039249. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. Effect of nonsteroidal anti-inflammatory agents on immune complex- and chemotactic factor-induced inflammation. Immunopharmacology. 1982 Jun;4(3):253–266. doi: 10.1016/0162-3109(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. The effect of nonsteroidal anti-inflammatory agents on E. coli-induced inflammation. Immunopharmacology. 1982 Feb;4(1):11–22. doi: 10.1016/0162-3109(82)90022-4. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980 Sep;41(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Price T. H., Beatty P. G., Corpuz S. R. In vivo inhibition of neutrophil function in the rabbit using monoclonal antibody to CD18. J Immunol. 1987 Dec 15;139(12):4174–4177. [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Rutledge B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986 Jan;136(2):649–654. [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]



