Abstract
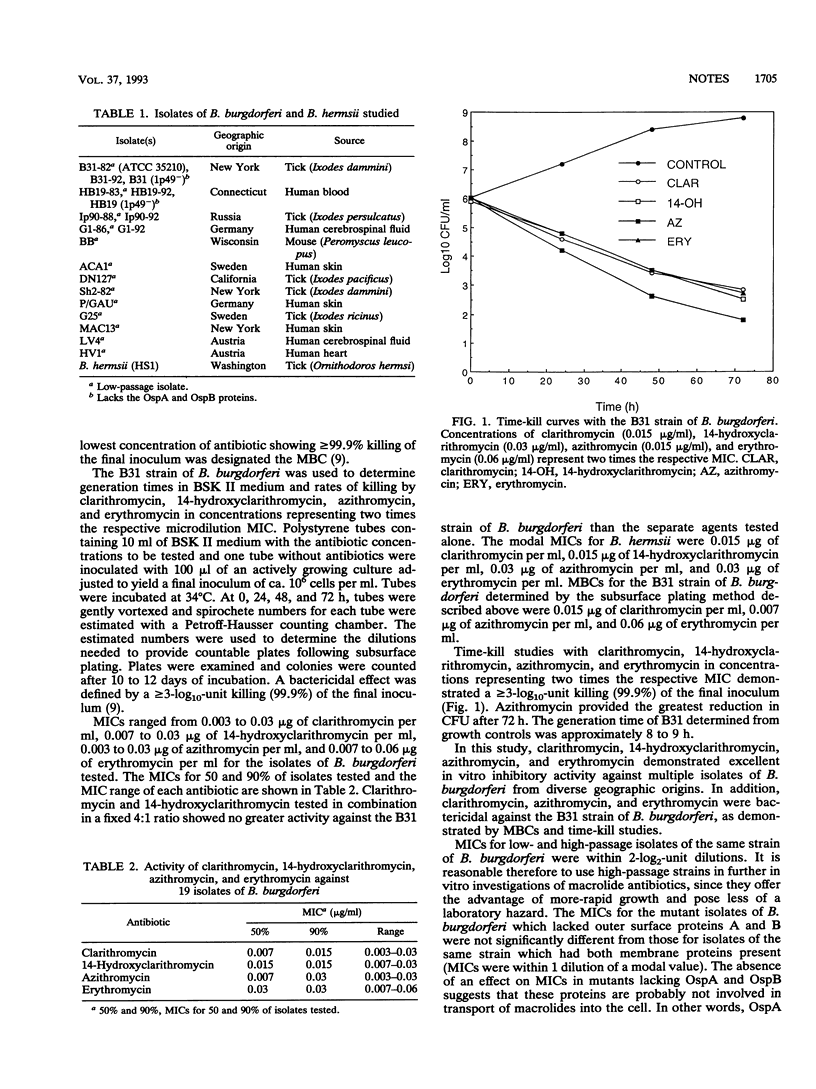
The in vitro activities of the macrolide antibiotics clarithromycin, 14-hydroxy-clarithromycin, azithromycin, and erythromycin against 19 isolates of Borrelia burgdorferi were investigated. MICs ranged from 0.003 to 0.03 microgram of clarithromycin per ml, 0.007 to 0.03 microgram of 14-hydroxyclarithromycin per ml, 0.003 to 0.03 microgram of azithromycin per ml, and 0.007 to 0.06 microgram of erythromycin per ml. Time-kill studies using the B31 strain of B. burgdorferi demonstrated a > or = 3-log10-unit killing after 72 h with each of the macrolide antibiotics tested in concentrations representing twice the respective MICs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dever L. L., Jorgensen J. H., Barbour A. G. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol. 1992 Oct;30(10):2692–2697. doi: 10.1128/jcm.30.10.2692-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Johnson R. C. Isolation techniques for spirochetes and their sensitivity to antibiotics in vitro and in vivo. Rev Infect Dis. 1989 Sep-Oct;11 (Suppl 6):S1505–S1510. doi: 10.1093/clinids/11.supplement_6.s1505. [DOI] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Gorevic P. D., Halperin J. J., Volkman D. J., Dattwyler R. J. A perspective on the treatment of Lyme borreliosis. Rev Infect Dis. 1989 Sep-Oct;11 (Suppl 6):S1518–S1525. doi: 10.1093/clinids/11.supplement_6.s1518. [DOI] [PubMed] [Google Scholar]
- Massarotti E. M., Luger S. W., Rahn D. W., Messner R. P., Wong J. B., Johnson R. C., Steere A. C. Treatment of early Lyme disease. Am J Med. 1992 Apr;92(4):396–403. doi: 10.1016/0002-9343(92)90270-l. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The development of macrolides: clarithromycin in perspective. J Antimicrob Chemother. 1991 Feb;27 (Suppl A):1–9. doi: 10.1093/jac/27.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G., Süss E., Gross B. Comparative antimicrobial activity of the new macrolides against Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):651–653. doi: 10.1007/BF01968150. [DOI] [PubMed] [Google Scholar]
- Rahn D. W., Malawista S. E. Lyme disease: recommendations for diagnosis and treatment. Ann Intern Med. 1991 Mar 15;114(6):472–481. doi: 10.7326/0003-4819-114-6-472. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Thompson P. A., Barbour A. G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993 Jan;167(1):165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Ballow C. H. Tissue-directed pharmacokinetics. Am J Med. 1991 Sep 12;91(3A):5S–11S. doi: 10.1016/0002-9343(91)90394-d. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Hutchinson G. J., Rahn D. W., Sigal L. H., Craft J. E., DeSanna E. T., Malawista S. E. Treatment of the early manifestations of Lyme disease. Ann Intern Med. 1983 Jul;99(1):22–26. doi: 10.7326/0003-4819-99-1-22. [DOI] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Outer membrane permeability barrier to azithromycin, clarithromycin, and roxithromycin in gram-negative enteric bacteria. Antimicrob Agents Chemother. 1993 Feb;37(2):354–356. doi: 10.1128/aac.37.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]


