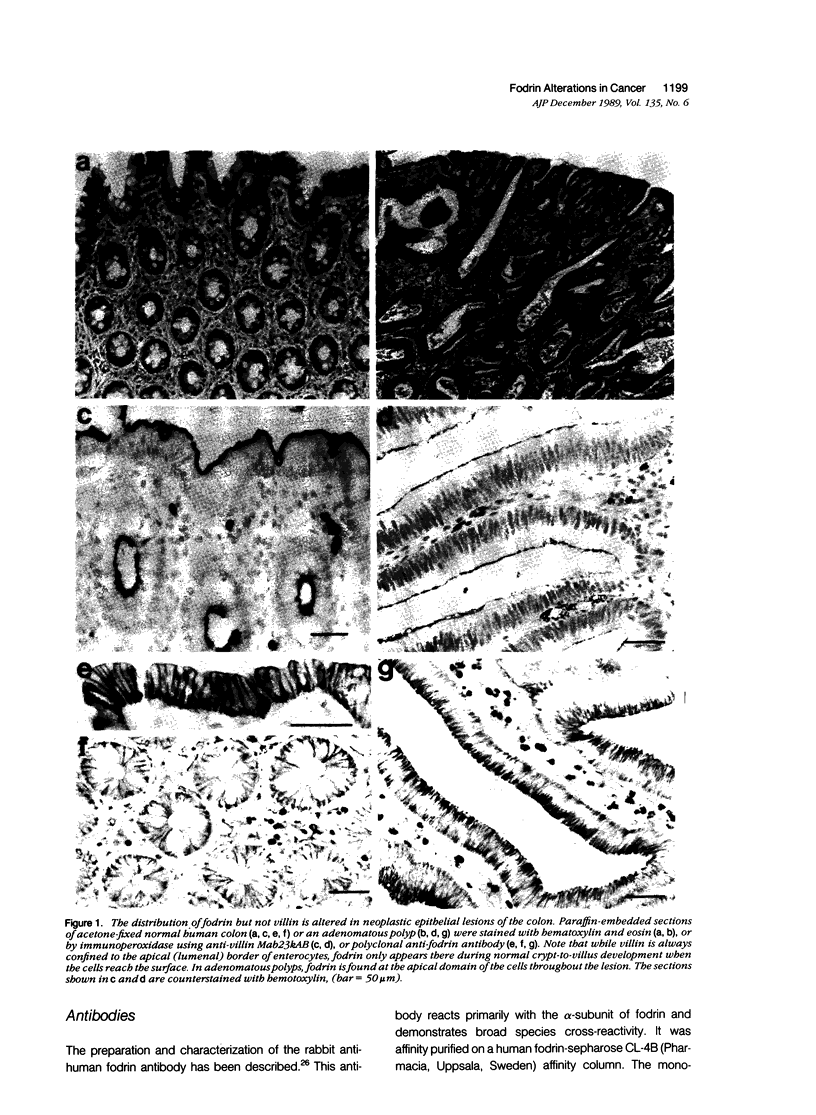
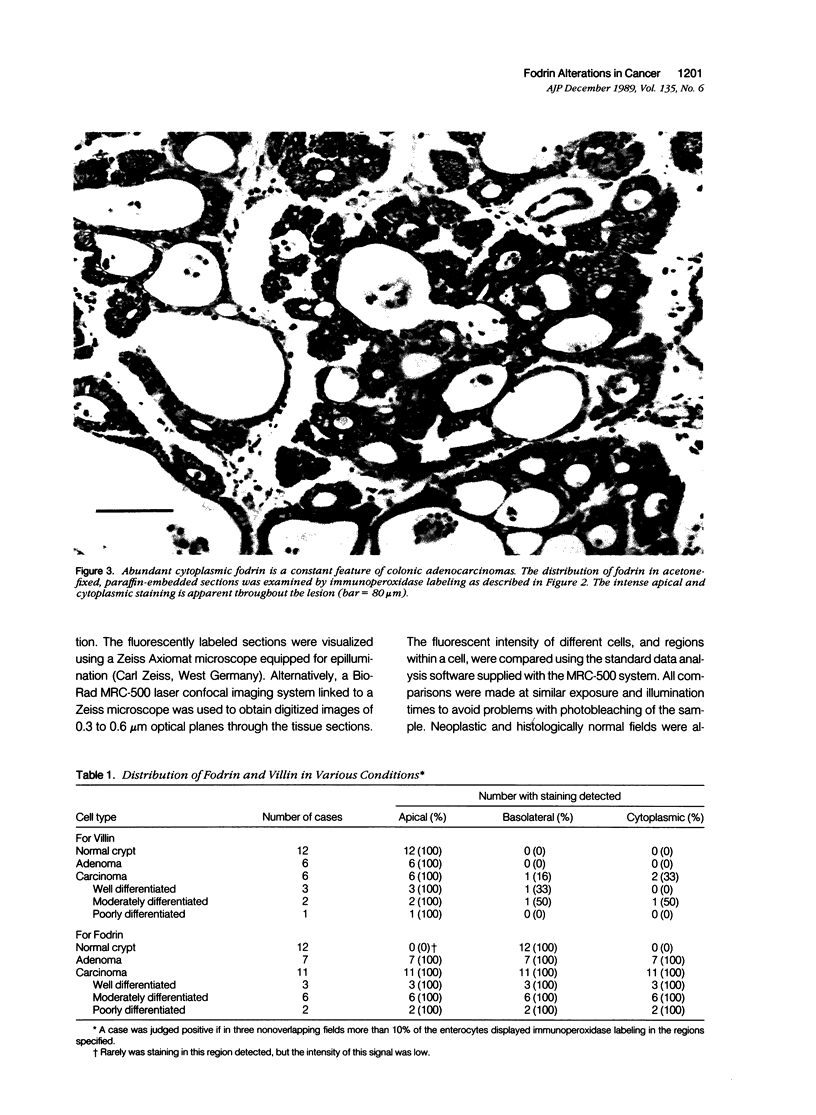
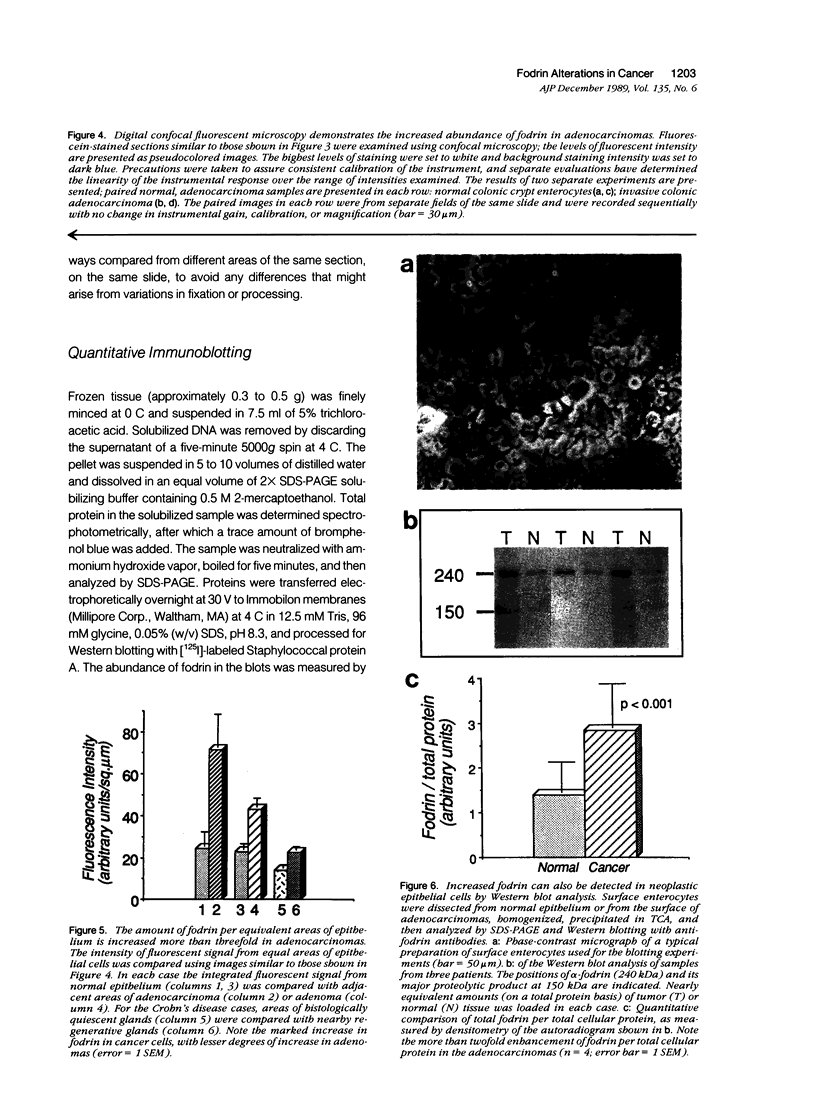
Abstract
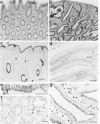
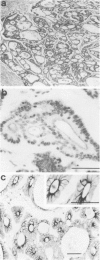
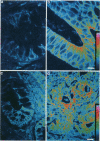
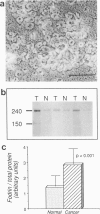
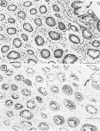
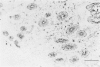
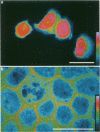
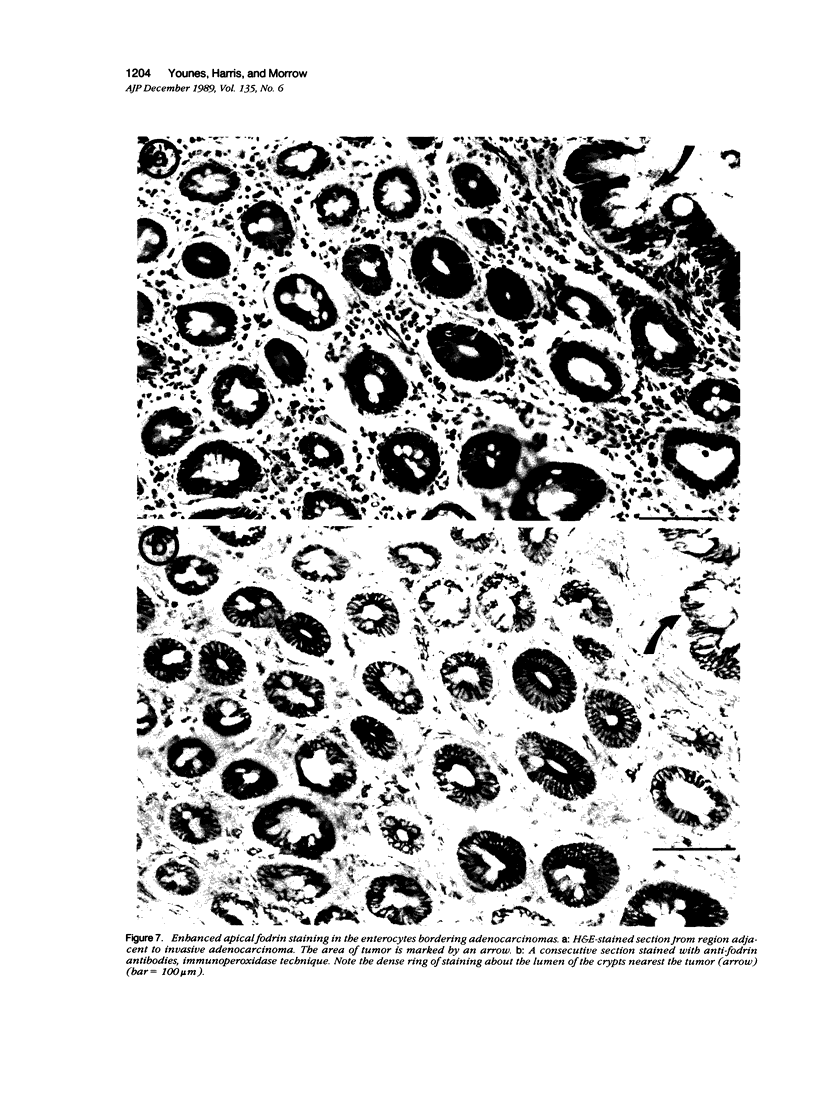
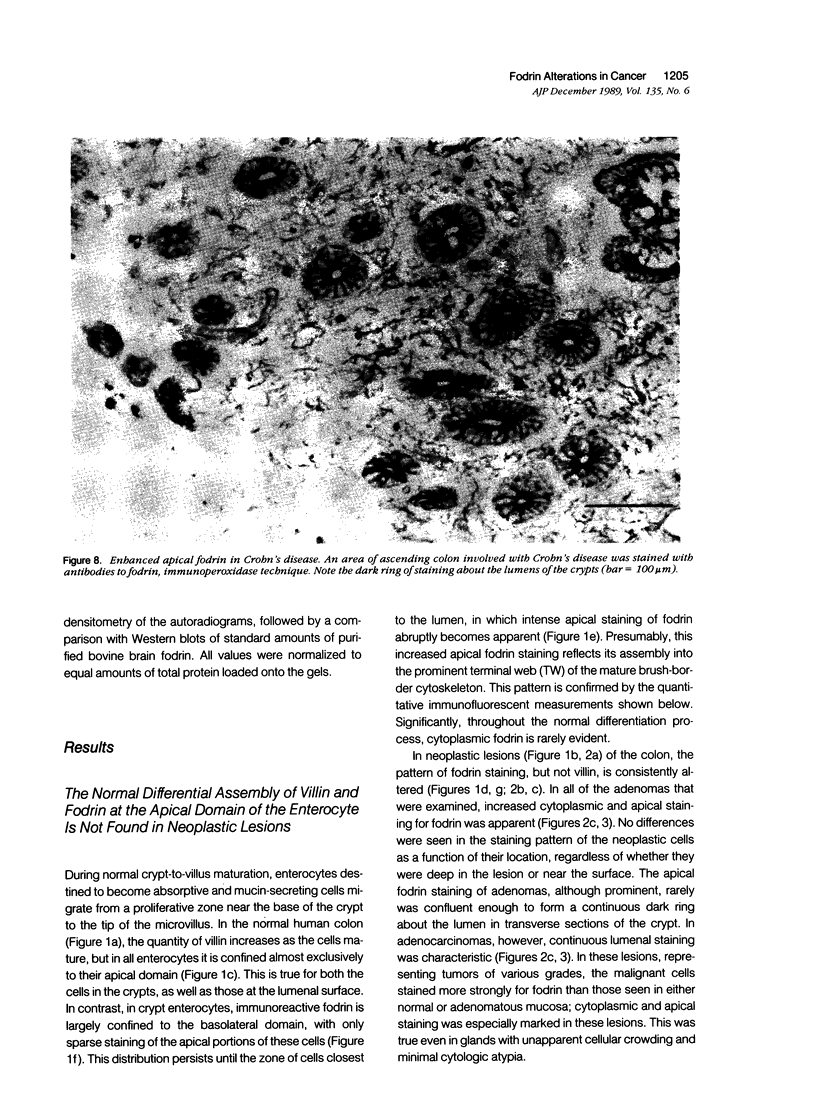
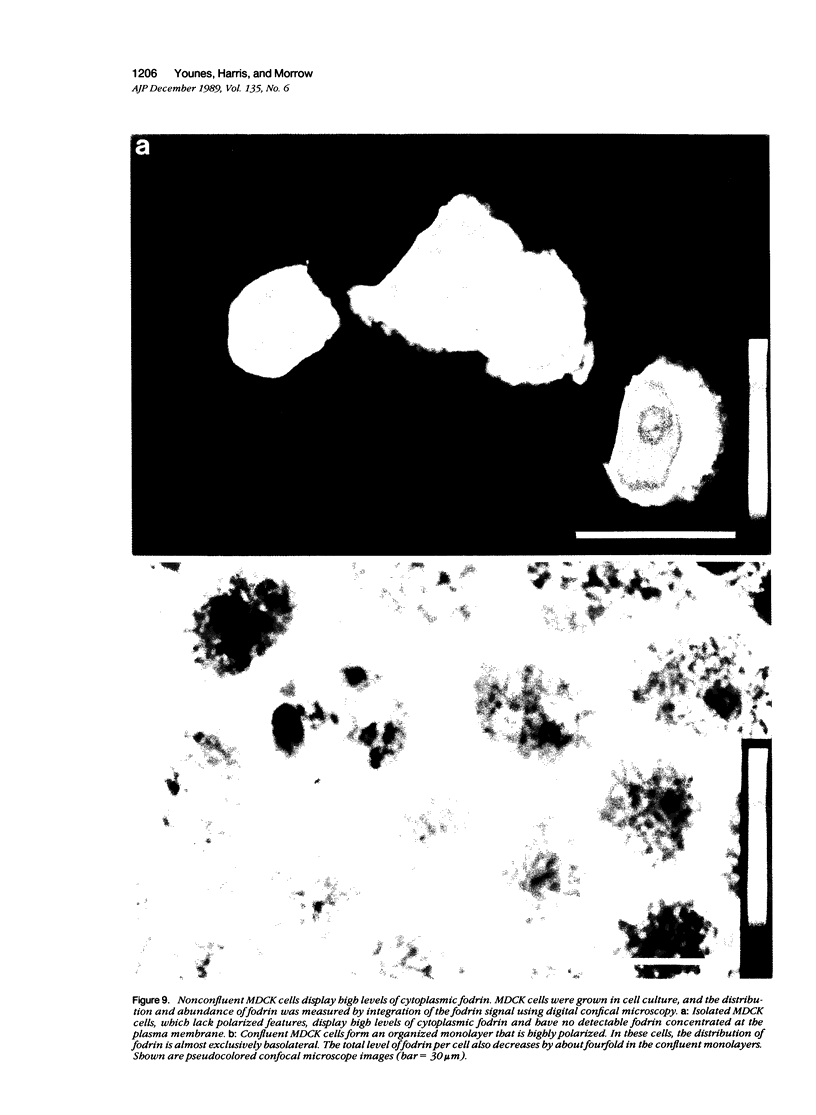
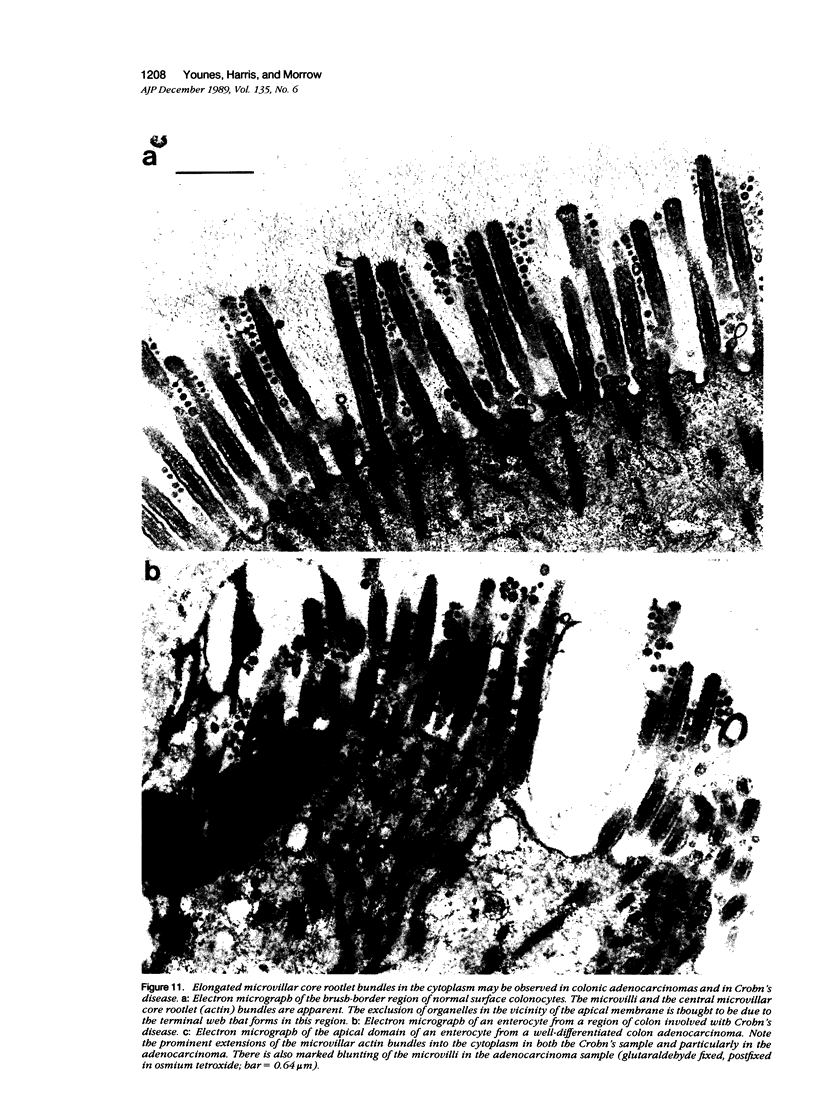
Fodrin (nonerythroid spectrin) is a 475,000 molecular weight (MW) (apparent) heterodimeric actin-binding protein usually found in mature cells at the cytoplasmic face of the plasma membrane. While its precise role is uncertain, it may participate in the establishment and/or maintenance of cell polarity, shape, and specialized receptor domains. In polarized epithelial cells, an asymmetric distribution of fodrin appears to signal phenotypic maturity. Using immunohistochemical techniques, the distribution of fodrin in enterocytes during normal crypt-to-villus maturation, and in adenomas, adenocarcinomas, and cultured Madin-Darby Canine Kidney (MDCK) cells has been studied and its abundance quantitated by immunoblotting and digital immunofluorescent confocal microscopy. During normal maturation, fodrin was found to assemble at the apex of the enterocyte, presumably in the terminal web, only in those cells near the villus tip. Villin was found in an apical location in both crypt and surface enterocytes. In adenocarcinomas of the colon (n = 11), there were enhanced levels of fodrin at the apex, and an approximately threefold increase in the total amount of fodrin per cell relative to normal crypt enterocytes. An increased percentage of this protein was also found in the cytoplasm. Adenomas (n = 7), nonconfluent MDCK cells in culture, and two (of two) cases of ductal carcinoma of the breast also demonstrated enhanced cytoplasmic and total fodrin. Supranormal levels of fodrin at the apex of enterocytes were also observed in Crohn's disease samples and in the normal-appearing enterocytes adjacent to a tumor. It is hypothesized that increased apical fodrin may signal a reaction of the microvillar brush border to pathologic stress, while increased cytoplasmic and total pools of fodrin may mark neoplastic activity. These findings may be of diagnostic value, particularly in the evaluation of small biopsies or cytologic material.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carboni J. M., Howe C. L., West A. B., Barwick K. W., Mooseker M. S., Morrow J. S. Characterization of intestinal brush border cytoskeletal proteins of normal and neoplastic human epithelial cells. A comparison with the avian brush border. Am J Pathol. 1987 Dec;129(3):589–600. [PMC free article] [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988 May 19;333(6170):272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Fishkind D. J., Mooseker M. S., Morrow J. S. Functional diversity among spectrin isoforms. Cell Motil Cytoskeleton. 1989;12(4):225–247. doi: 10.1002/cm.970120405. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Baron M. D., Critchley D. R., Wootton J. C. Structural analysis of homologous repeated domains in alpha-actinin and spectrin. Int J Biol Macromol. 1989 Apr;11(2):81–90. doi: 10.1016/0141-8130(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Giebelhaus D. H., Zelus B. D., Henchman S. K., Moon R. T. Changes in the expression of alpha-fodrin during embryonic development of Xenopus laevis. J Cell Biol. 1987 Aug;105(2):843–853. doi: 10.1083/jcb.105.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Helmchen U., Osborn M. Villin--a marker of brush border differentiation and cellular origin in human renal cell carcinoma. Am J Pathol. 1986 Aug;124(2):294–302. [PMC free article] [PubMed] [Google Scholar]
- Harris A. S., Green L. A., Ainger K. J., Morrow J. S. Mechanism of cytoskeletal regulation (I): functional differences correlate with antigenic dissimilarity in human brain and erythrocyte spectrin. Biochim Biophys Acta. 1985 Aug 8;830(2):147–158. doi: 10.1016/0167-4838(85)90022-6. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Bokoch G. M., Tolley J. O., Allen R. A. Lateral segregation of neutrophil chemotactic receptors into actin- and fodrin-rich plasma membrane microdomains depleted in guanyl nucleotide regulatory proteins. J Cell Biol. 1988 Sep;107(3):921–928. doi: 10.1083/jcb.107.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob R., Zimmermann M., Schoner W., Drenckhahn D. Colocalization and coprecipitation of ankyrin and Na+,K+-ATPase in kidney epithelial cells. Eur J Cell Biol. 1988 Feb;45(2):230–237. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louvard D. The function of the major cytoskeletal components of the brush border. Curr Opin Cell Biol. 1989 Feb;1(1):51–57. doi: 10.1016/s0955-0674(89)80036-5. [DOI] [PubMed] [Google Scholar]
- Moll R., Robine S., Dudouet B., Louvard D. Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(3):155–169. doi: 10.1007/BF02899208. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Cianci C. D., Ardito T., Mann A. S., Kashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989 Feb;108(2):455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S. The spectrin membrane skeleton: emerging concepts. Curr Opin Cell Biol. 1989 Feb;1(1):23–29. doi: 10.1016/s0955-0674(89)80032-8. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987 Aug 6;328(6130):533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1986 Nov;103(5):1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin D., Langley O. K., Aunis D. Anti-alpha-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987 Apr 2;326(6112):498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- Robson R. M. Intermediate filaments. Curr Opin Cell Biol. 1989 Feb;1(1):36–43. doi: 10.1016/s0955-0674(89)80034-1. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Haffen K. Developmental pattern of calmodulin-binding proteins in rat jejunal epithelial cells. Differentiation. 1987;35(3):219–227. doi: 10.1111/j.1432-0436.1987.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Lacroix B., Haffen K., Kedinger M. Expression of brush border calmodulin-binding proteins during human small and large bowel differentiation. Cell Differ. 1988 Jul;24(2):119–131. doi: 10.1016/0045-6039(88)90063-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Shibayama T., Carboni J. M., Mooseker M. S. Assembly of the intestinal brush border: appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987 Jul;105(1):335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986 Sep;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Masaki T., Hirokawa N. Developmental organization of the intestinal brush-border cytoskeleton. Cell Motil Cytoskeleton. 1988;9(4):299–311. doi: 10.1002/cm.970090403. [DOI] [PubMed] [Google Scholar]
- Wasenius V. M., Saraste M., Salvén P., Erämaa M., Holm L., Lehto V. P. Primary structure of the brain alpha-spectrin. J Cell Biol. 1989 Jan;108(1):79–93. doi: 10.1083/jcb.108.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. B., Isaac C. A., Carboni J. M., Morrow J. S., Mooseker M. S., Barwick K. W. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988 Feb;94(2):343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]