Abstract
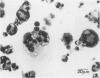
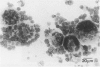
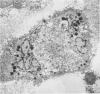
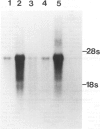
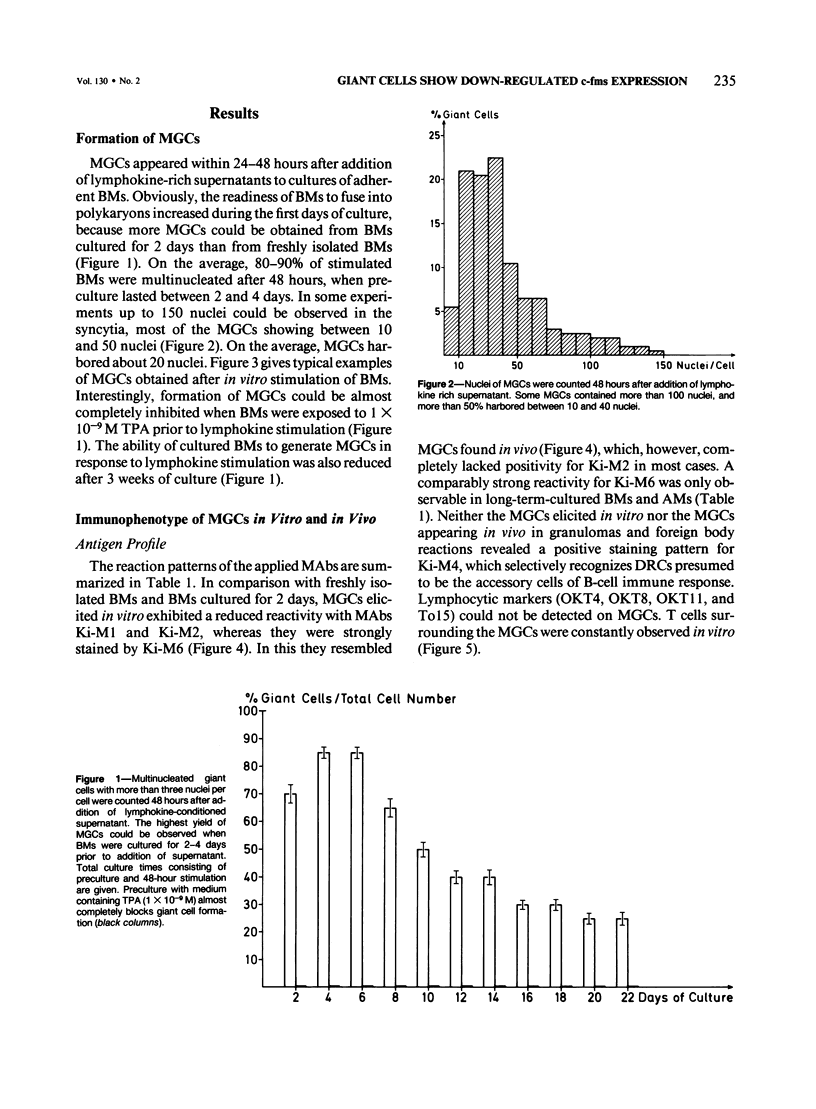
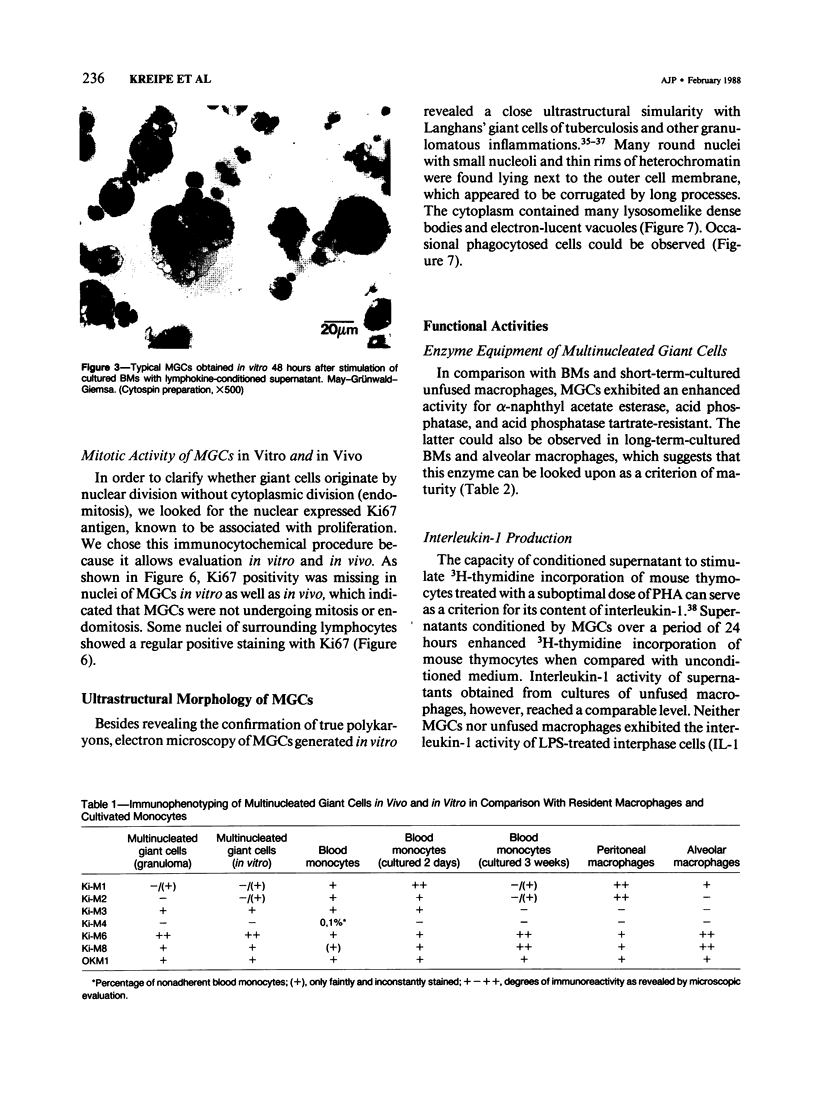
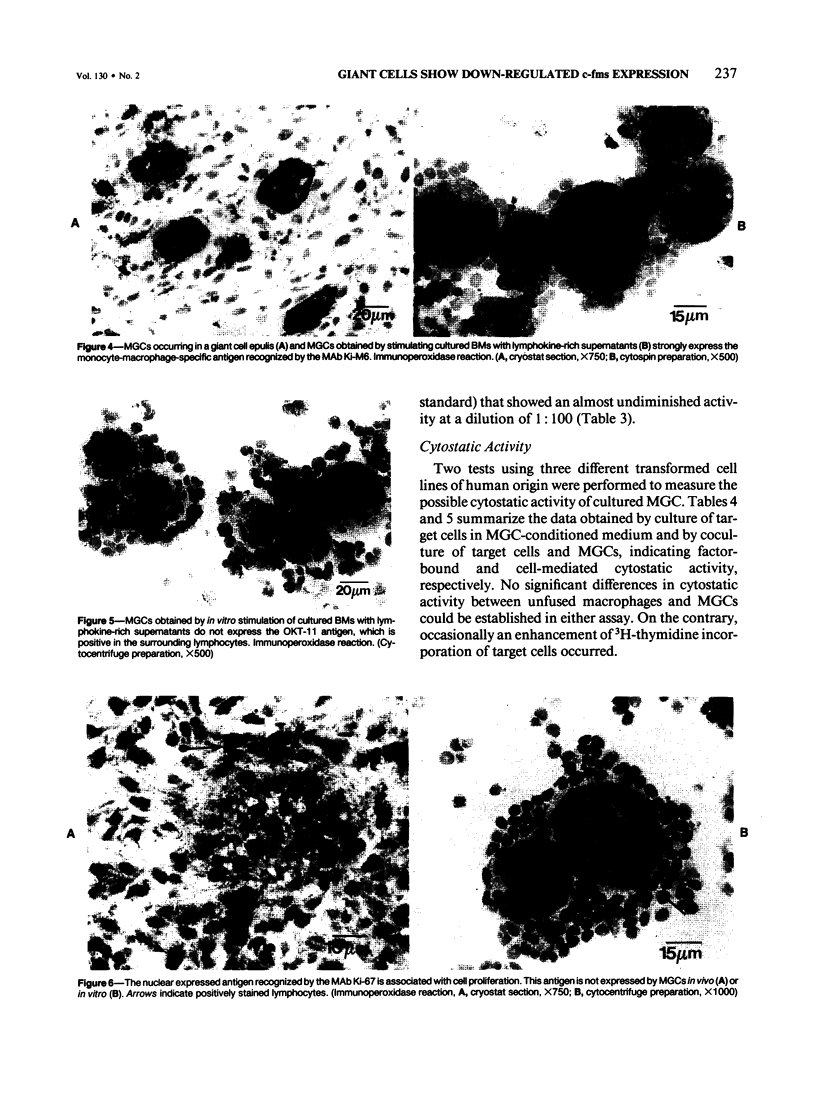
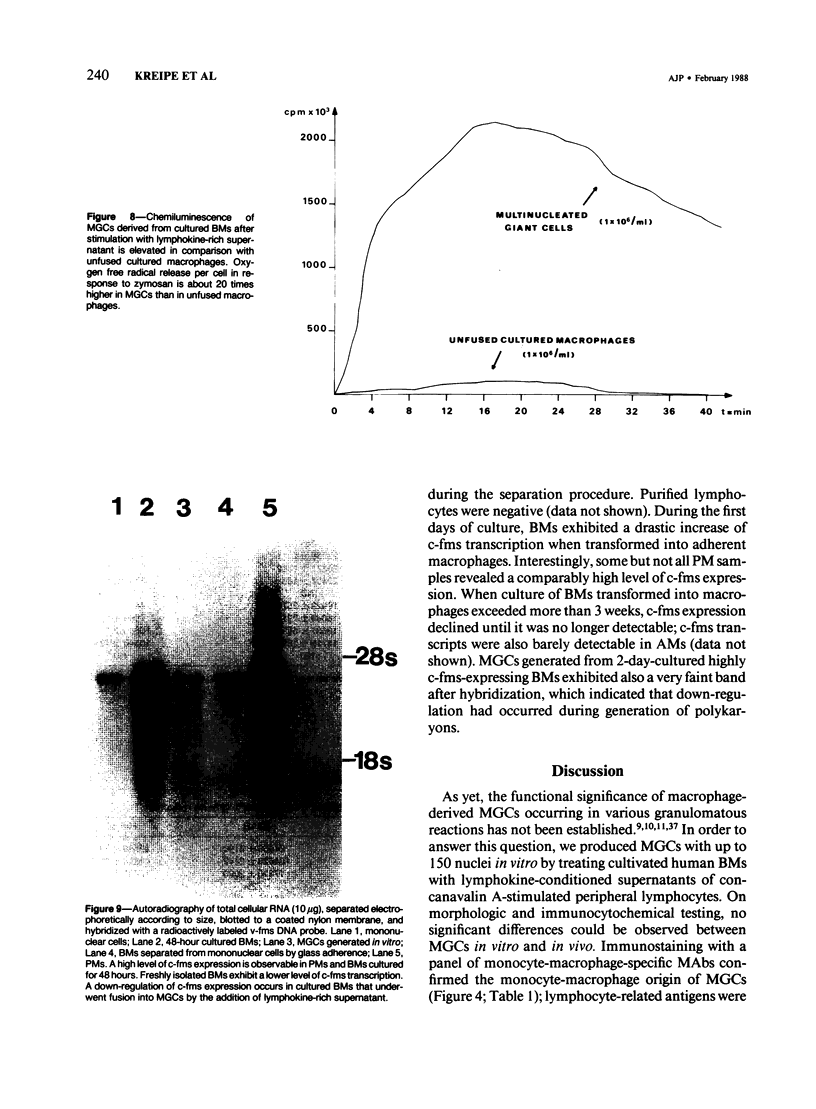
Although multinucleated giant cells (MGCs) are a known feature of granulomatous reactions, little is known about their destination and function. In this study human blood monocyte (BM)-derived giant cells were generated by lymphokine stimulation in vitro. Their immunophenotype and ultrastructural morphology resembled that of MGCs occurring in vivo. Mitotic activity within MGCs could not be established either in vitro or in vivo. Enzyme equipment of MGCs was elevated in comparison with monocyte-macrophages. In comparison with unfused monocyte-macrophages, MGCs did not reveal a higher level of interleukin-1 production or cytostatic activity. They showed, however, a 20-30-fold increase in the production of oxygen-free radicals in response to zymosan. Transcription of the proto-oncogene c-fms was enhanced in short-term cultivated BM and was rapidly down-regulated in MGCs after fusion had occurred. It is concluded that MGCs represent highly stimulated cells of monocyte-macrophage lineage at a terminal stage of maturation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1986;69(3-4):253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cain H., Kraus B. Cellular aspects of granulomas. Pathol Res Pract. 1982 Oct;175(1):13–37. doi: 10.1016/S0344-0338(82)80040-X. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaili J. H., Hafez G. R., Warner T. F. Anaplastic carcinoma of the thyroid with osteoclast-like giant cells. Cancer. 1983 Dec 1;52(11):2122–2128. doi: 10.1002/1097-0142(19831201)52:11<2122::aid-cncr2820521125>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987 Apr;69(4):1259–1261. [PubMed] [Google Scholar]
- Ichijima K., Kobashi Y., Ueda Y., Matsuo S. Breast cancer with reactive multinucleated giant cells: report of three cases. Acta Pathol Jpn. 1986 Mar;36(3):449–457. doi: 10.1111/j.1440-1827.1986.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Castriotta R., Yoshida T. Strain variation of bacillus Calmette-Guerin-induced pulmonary granuloma formation is correlated with anergy and the local production of migration inhibition factor and interleukin 1. Am J Pathol. 1985 May;119(2):223–235. [PMC free article] [PubMed] [Google Scholar]
- Kreipe H., Radzun H. J., Parwaresch M. R. Phenotypic differentiation patterns of the human monocyte/macrophage system. Histochem J. 1986 Aug;18(8):441–450. doi: 10.1007/BF01675337. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Pelus L. M., Ralph P., Bockman R. S., Moore M. A. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci U S A. 1979 May;76(5):2326–2330. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Gordon S. Secretion of plasminogen activator by bone marrow-derived mononuclear phagocytes and its enhancement by colony-stimulating factor. J Exp Med. 1979 Aug 1;150(2):231–245. doi: 10.1084/jem.150.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- Matthews N. Human monocyte cytotoxin is not identical with lymphoblastoid lymphotoxin. Eur J Immunol. 1985 Mar;15(3):311–313. doi: 10.1002/eji.1830150321. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Papadimitriou J. M., Robertson T. A., Walters M. N. An analysis of the Phagocytic potential of multinucleate foreign body giant cells. Am J Pathol. 1975 Feb;78(2):343–358. [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Sforsina D., Papaelias L. Kinetics of multinucleate giant cell formation and their modification by various agents in foreign body reactions. Am J Pathol. 1973 Nov;73(2):349–364. [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., van Bruggen I. Evidence that multinucleate giant cells are examples of mononuclear phagocytic differentiation. J Pathol. 1986 Feb;148(2):149–157. doi: 10.1002/path.1711480205. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Dommes M. The homogeneity and monocytic origin of human peritoneal macrophages evidence by comparison of esterase polymorphism. Am J Pathol. 1981 Feb;102(2):209–218. [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Feller A. C., Peters K. P., Hansmann M. L. Peroxidase-positive mononuclear leukocytes as possible precursors of human dendritic reticulum cells. J Immunol. 1983 Dec;131(6):2719–2725. [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Kreipe H., Hansmann M. L., Barth J. Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol. 1986 Oct;125(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- Poste G. The tumoricidal properties of inflammatory tissue macrophages and multinucleate giant cells. Am J Pathol. 1979 Aug;96(2):595–610. [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Jackson B. K., Beachey E. H., Kang A. H. Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen-and mitogen-stimulated lymphocytes. J Exp Med. 1982 Jan 1;155(1):168–178. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzun H. J., Kreipe H., Parwaresch M. R. Tartrate-resistant acid phosphatase as a differentiation marker for the human mononuclear phagocyte system. Hematol Oncol. 1983 Oct-Dec;1(4):321–327. doi: 10.1002/hon.2900010404. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Feller A. C., Hansmann M. L. Monocyte/macrophage-specific monoclonal antibody Ki-M1 recognizes interdigitating reticulum cells. Am J Pathol. 1984 Dec;117(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Kreipe H. Monocytic origin of human alveolar macrophages. J Histochem Cytochem. 1983 Feb;31(2):318–324. doi: 10.1177/31.2.6833743. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Rettenmier C. W., Sacca R., Furman W. L., Roussel M. F., Holt J. T., Nienhuis A. W., Stanley E. R., Sherr C. J. Expression of the human c-fms proto-oncogene product (colony-stimulating factor-1 receptor) on peripheral blood mononuclear cells and choriocarcinoma cell lines. J Clin Invest. 1986 Jun;77(6):1740–1746. doi: 10.1172/JCI112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Griffin J., Kufe D. W. Effects of interferon-gamma on proto-oncogene expression during induction of human monocytic differentiation. J Immunol. 1987 Mar 15;138(6):1954–1958. [PubMed] [Google Scholar]
- Schlesinger L., Musson R. A., Johnston R. B., Jr Functional and biochemical studies of multinucleated giant cells derived from the culture of human monocytes. J Exp Med. 1984 Apr 1;159(4):1289–1294. doi: 10.1084/jem.159.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shulman L. N., Robinson S. H. Origin of multinucleated giant cells in long-term diffusion chamber cultures. Proc Soc Exp Biol Med. 1982 Jul;170(3):359–362. doi: 10.3181/00379727-170-41442. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Stein H., Uchánska-Ziegler B., Gerdes J., Ziegler A., Wernet P. Hodgkin and Sternberg-Reed cells contain antigens specific to late cells of granulopoiesis. Int J Cancer. 1982 Mar 15;29(3):283–290. doi: 10.1002/ijc.2910290310. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel A. H., Hadden J. W. Lymphokine-mediated fusion and migration inhibition of alveolar macrophages. Exp Mol Pathol. 1980 Oct;33(2):153–168. doi: 10.1016/0014-4800(80)90016-7. [DOI] [PubMed] [Google Scholar]
- Warfel A. H. Macrophage fusion and multinucleated giant cell formation, surface morphology. Exp Mol Pathol. 1978 Apr;28(2):163–176. doi: 10.1016/0014-4800(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Hobbs M. M., Misukonis M. A. Recombinant human gamma-interferon induces human monocyte polykaryon formation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4554–4557. doi: 10.1073/pnas.81.14.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rhee H. J., van der Burgh-de Winter C. P., Daems W. T. The differentiation of monocytes into macrophages, epithelioid cells, and multinucleated giant cells in subcutaneous granulomas. I. Fine structure. Cell Tissue Res. 1979 Apr 12;197(3):355–378. doi: 10.1007/BF00233563. [DOI] [PubMed] [Google Scholar]