Abstract
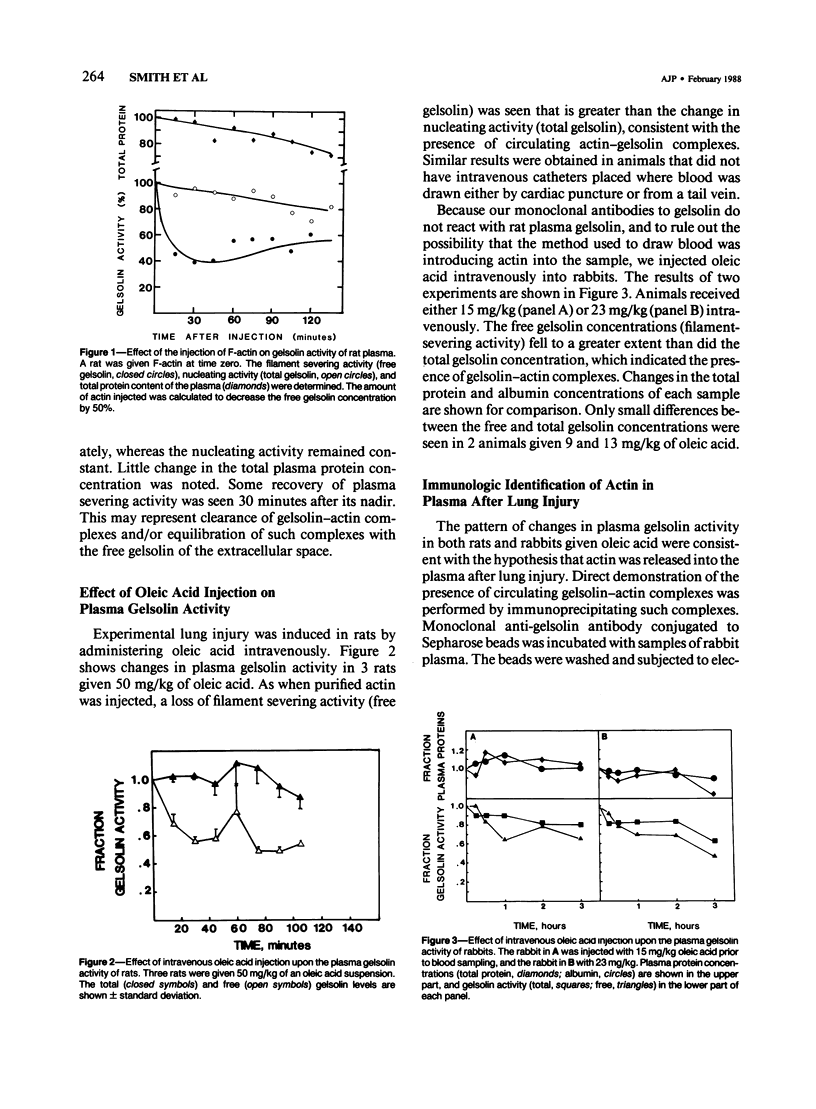
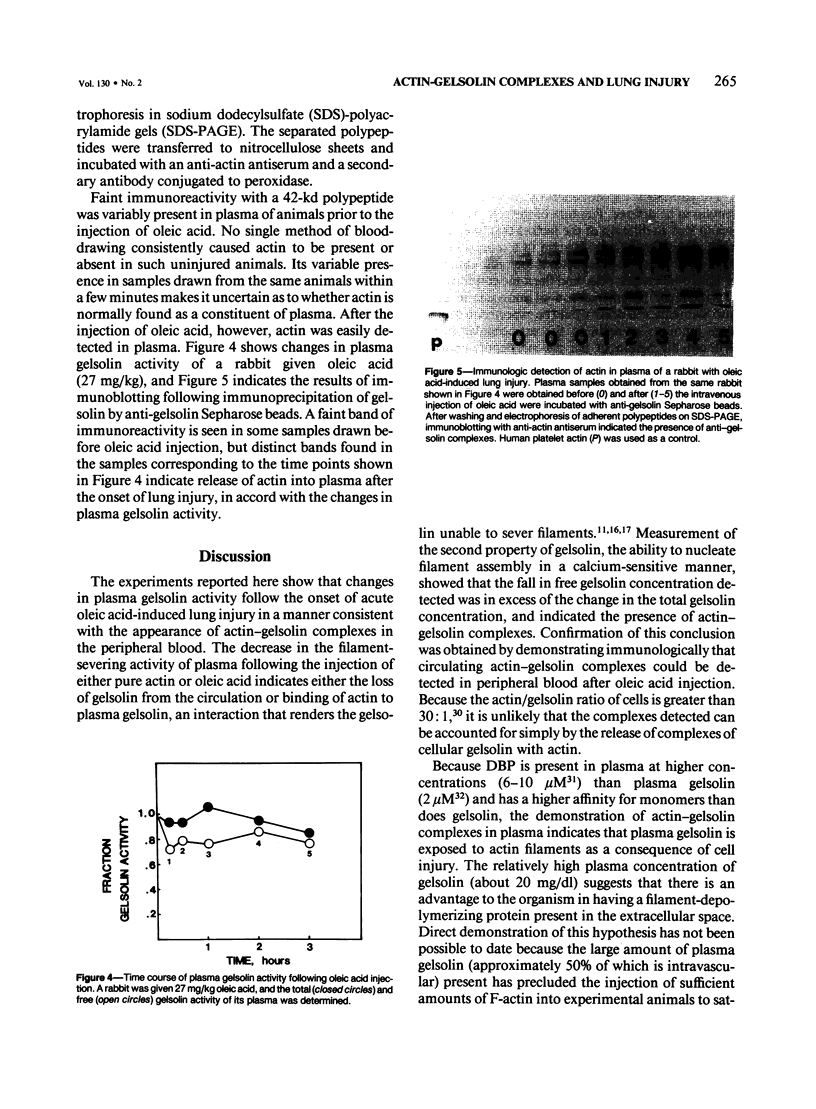
Plasma gelsolin is one of two extracellular proteins that bind actin, a major body protein, with high affinity. The authors performed a series of experiments to determine whether tissue injury leads to actin release and the formation of circulating actin-gelsolin complexes. Two functions of plasma gelsolin, filament-nucleating and filament-severing activity, were used to measure total and free gelsolin concentrations, respectively. Both gelsolin and gelsolin-actin complexes nucleate actin assembly, whereas only free gelsolin severs actin filaments. Therefore, nucleation reflects the total gelsolin concentration, severing, the free gelsolin concentration, and the difference, gelsolin-actin complexes. Injection of F-actin in the rat caused a reduction in the free, but not total, gelsolin levels, consistent with the formation of circulating actin-gelsolin complexes. Oleic acid (50 mg/kg) administered intravenously in rats, a treatment that causes acute hemorrhagic pulmonary necrosis, caused the free gelsolin concentration to fall to a greater extent than the total gelsolin concentration, which indicated the presence of circulating actin-gelsolin complexes. Lower doses (9-27 mg/kg) in rabbits caused a qualitatively similar but smaller change in the free gelsolin level. Plasma gelsolin was immunoprecipitated at times when actin-gelsolin complexes were present, as determined functionally, and bound actin was demonstrated by immunoblotting with an anti-actin antiserum. These studies show that considerable amounts of actin are released into the extracellular space during acute lung injury and that circulating actin-gelsolin complexes can be detected in the peripheral blood.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan J., Coluccio L. M. Kinetic analysis of F-actin depolymerization in the presence of platelet gelsolin and gelsolin-actin complexes. J Cell Biol. 1985 Oct;101(4):1236–1244. doi: 10.1083/jcb.101.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C., Borgia R., Rungger-Brändle E., Weil R., Gabbiani G. An actin-destabilizing factor is present in human plasma. Experientia. 1979 Aug 15;35(8):1039–1041. doi: 10.1007/BF01949928. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Janmey P. A., Yin H. L. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986 Oct;103(4):1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coué M., Korn E. D. Interaction of plasma gelsolin with ADP-actin. J Biol Chem. 1986 Mar 15;261(8):3628–3631. [PubMed] [Google Scholar]
- De Scheerder I., Vandekerckhove J., Robbrecht J., Algoed L., De Buyzere M., De Langhe J., De Schrijver G., Clement D. Post-cardiac injury syndrome and an increased humoral immune response against the major contractile proteins (actin and myosin). Am J Cardiol. 1985 Oct 1;56(10):631–633. doi: 10.1016/0002-9149(85)91024-0. [DOI] [PubMed] [Google Scholar]
- Dickey B. F., Thrall R. S., McCormick J. R., Ward P. A. Oleic-acid-induced lung injury in the rat. Failure of indomethacin treatment or complement depletion to ablate lung injury. Am J Pathol. 1981 Jun;103(3):376–383. [PMC free article] [PubMed] [Google Scholar]
- Emerson D. L., Arnaud P., Galbraith R. M. Evidence of increased Gc:actin complexes in pregnant serum: a possible result of trophoblast embolism. Am J Reprod Immunol. 1983 Dec;4(4):185–189. doi: 10.1111/j.1600-0897.1983.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Lamelin J. P., Vassalli P., Majno G., Bouvier C. A., Cruchaud A., Lüscher E. F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973 Sep;72(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G. Human serum binding protein for vitamin D and its metabolites (DBP): evidence that actin is the DBP binding component in human skeletal muscle. Arch Biochem Biophys. 1982 Feb;213(2):538–544. doi: 10.1016/0003-9861(82)90581-1. [DOI] [PubMed] [Google Scholar]
- Harper K. D., McLeod J. F., Kowalski M. A., Haddad J. G. Vitamin D binding protein sequesters monomeric actin in the circulation of the rat. J Clin Invest. 1987 May;79(5):1365–1370. doi: 10.1172/JCI112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. E., Bamburg J. R., Weeds A. G. Actin filament disassembly in blood plasma. FEBS Lett. 1980 Nov 17;121(1):175–177. doi: 10.1016/0014-5793(80)81291-9. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Lind S. E. Capacity of human serum to depolymerize actin filaments. Blood. 1987 Aug;70(2):524–530. [PubMed] [Google Scholar]
- Janmey P. A., Lind S. E., Yin H. L., Stossel T. P. Effects of semi-dilute actin solutions on the mobility of fibrin protofibrils during clot formation. Biochim Biophys Acta. 1985 Aug 16;841(2):151–158. doi: 10.1016/0304-4165(85)90016-9. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Kinetics of actin monomer exchange at the slow growing ends of actin filaments and their relation to the elongation of filaments shortened by gelsolin. J Muscle Res Cell Motil. 1986 Oct;7(5):446–454. doi: 10.1007/BF01753587. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P., Lind S. E. Sequential binding of actin monomers to plasma gelsolin and its inhibition by vitamin D-binding protein. Biochem Biophys Res Commun. 1986 Apr 14;136(1):72–79. doi: 10.1016/0006-291x(86)90878-8. [DOI] [PubMed] [Google Scholar]
- Kurth M. C., Wang L. L., Dingus J., Bryan J. Purification and characterization of a gelsolin-actin complex from human platelets. Evidence for Ca2+-insensitive functions. J Biol Chem. 1983 Sep 25;258(18):10895–10903. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Emerson D. L., Werner P. A., Arnaud P., Goldschmidt-Clermont P., Galbraith R. M. Decreased serum group-specific component protein levels and complexes with actin in fulminant hepatic necrosis. Hepatology. 1985 Mar-Apr;5(2):271–275. doi: 10.1002/hep.1840050220. [DOI] [PubMed] [Google Scholar]
- Lees A., Haddad J. G., Lin S. Brevin and vitamin D binding protein: comparison of the effects of two serum proteins on actin assembly and disassembly. Biochemistry. 1984 Jun 19;23(13):3038–3047. doi: 10.1021/bi00308a030. [DOI] [PubMed] [Google Scholar]
- Lidman K., Biberfeld G., Fagraeus A., Norberg R., Torstensson R., Utter G., Carlsson L., Luca J., Lindberg U. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976 May;24(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- Lind S. E., Janmey P. A., Chaponnier C., Herbert T. J., Stossel T. P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987 Aug;105(2):833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind S. E., Smith D. B., Janmey P. A., Stossel T. P. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J Clin Invest. 1986 Sep;78(3):736–742. doi: 10.1172/JCI112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg R., Thorstensson R., Utter G., Fagraeus A. F-Actin-depolymerizing activity of human serum. Eur J Biochem. 1979 Oct 15;100(2):575–583. doi: 10.1111/j.1432-1033.1979.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Janmey P. A., Herbert T. J., Lind S. E. Quantitative measurement of plasma gelsolin and its incorporation into fibrin clots. J Lab Clin Med. 1987 Aug;110(2):189–195. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P. Contractile proteins in cell structure and function. Annu Rev Med. 1978;29:427–457. doi: 10.1146/annurev.me.29.020178.002235. [DOI] [PubMed] [Google Scholar]
- Thorstensson R., Utter G., Norberg R. Further characterization of the Ca2+-dependent F-actin-depolymerizing protein of human serum. Eur J Biochem. 1982 Aug;126(1):11–16. doi: 10.1111/j.1432-1033.1982.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980 Mar 25;255(6):2270–2272. [PubMed] [Google Scholar]
- Walsh P. G., Haddad J. G. "Rocket" immunoelectrophoresis assay of vitamin D-binding protein (Gc globulin) in human serum. Clin Chem. 1982 Aug;28(8):1781–1783. [PubMed] [Google Scholar]
- Yin H. L., Kwiatkowski D. J., Mole J. E., Cole F. S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J Biol Chem. 1984 Apr 25;259(8):5271–5276. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Young W. O., Goldschmidt-Clermont P. J., Emerson D. L., Lee W. M., Jollow D. J., Galbraith R. M. Correlation between extent of liver damage in fulminant hepatic necrosis and complexing of circulating group-specific component (vitamin D-binding protein). J Lab Clin Med. 1987 Jul;110(1):83–90. [PubMed] [Google Scholar]



