Abstract
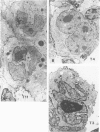
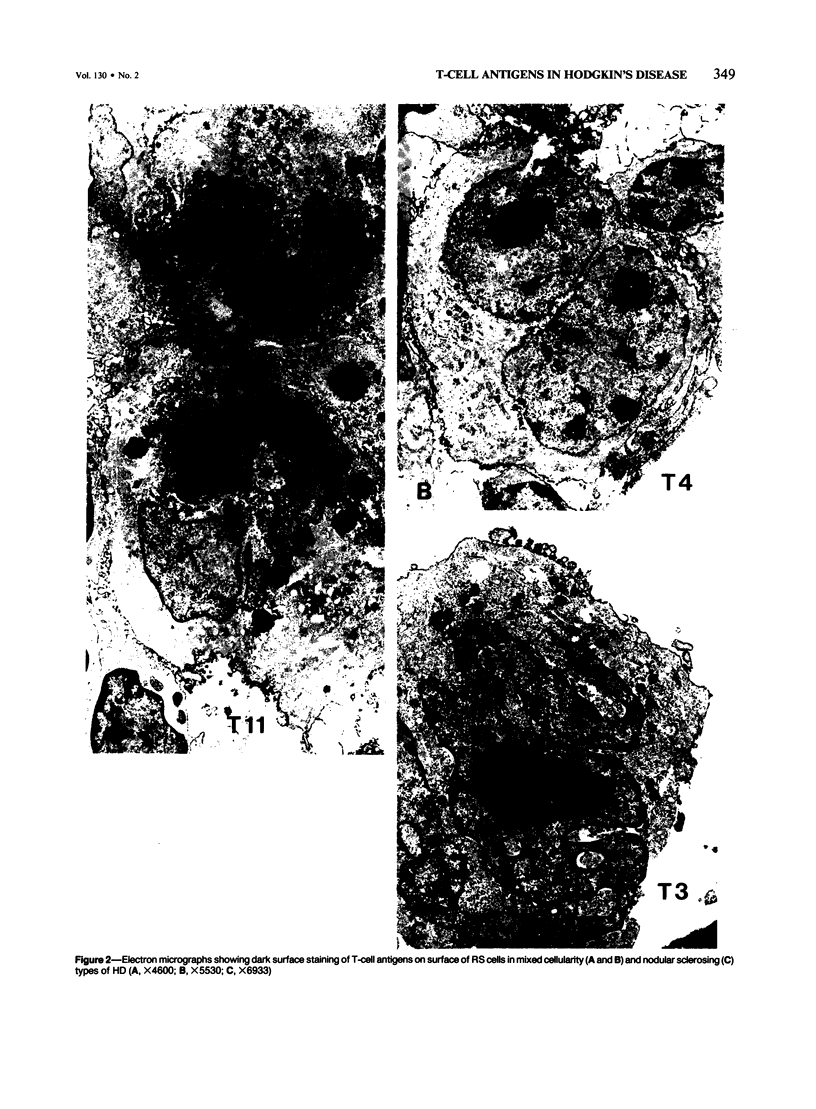
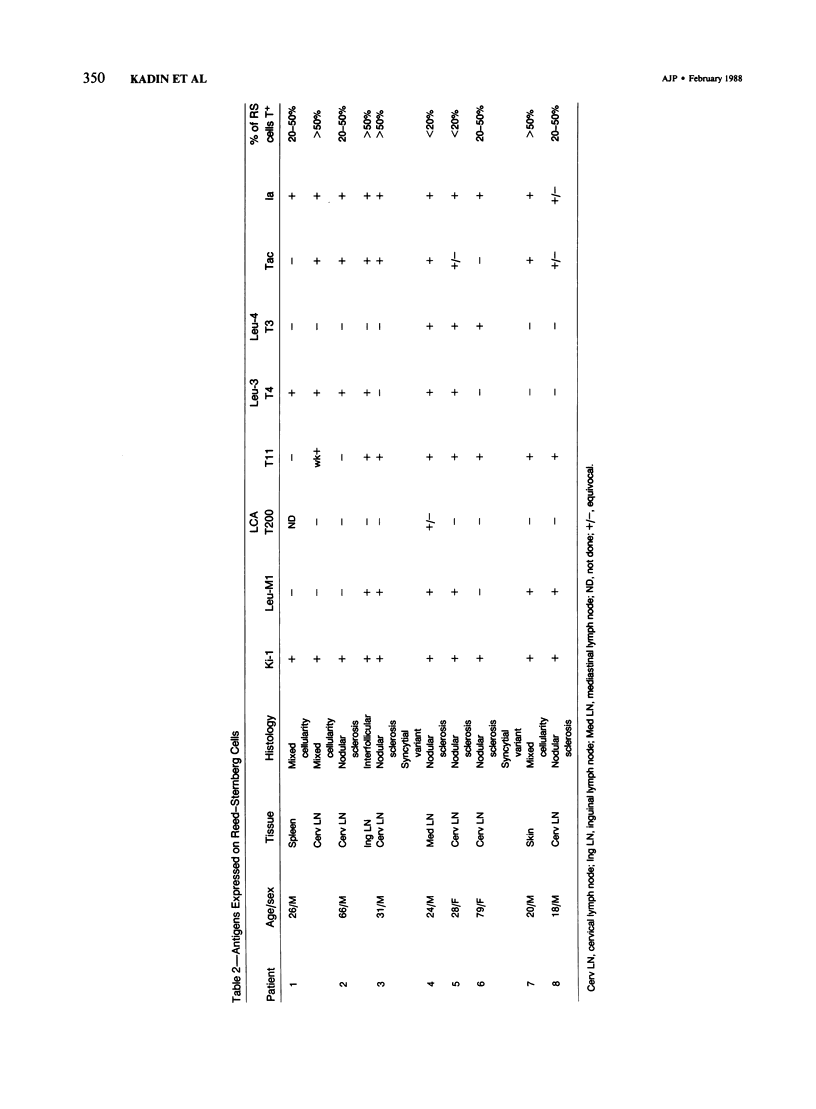
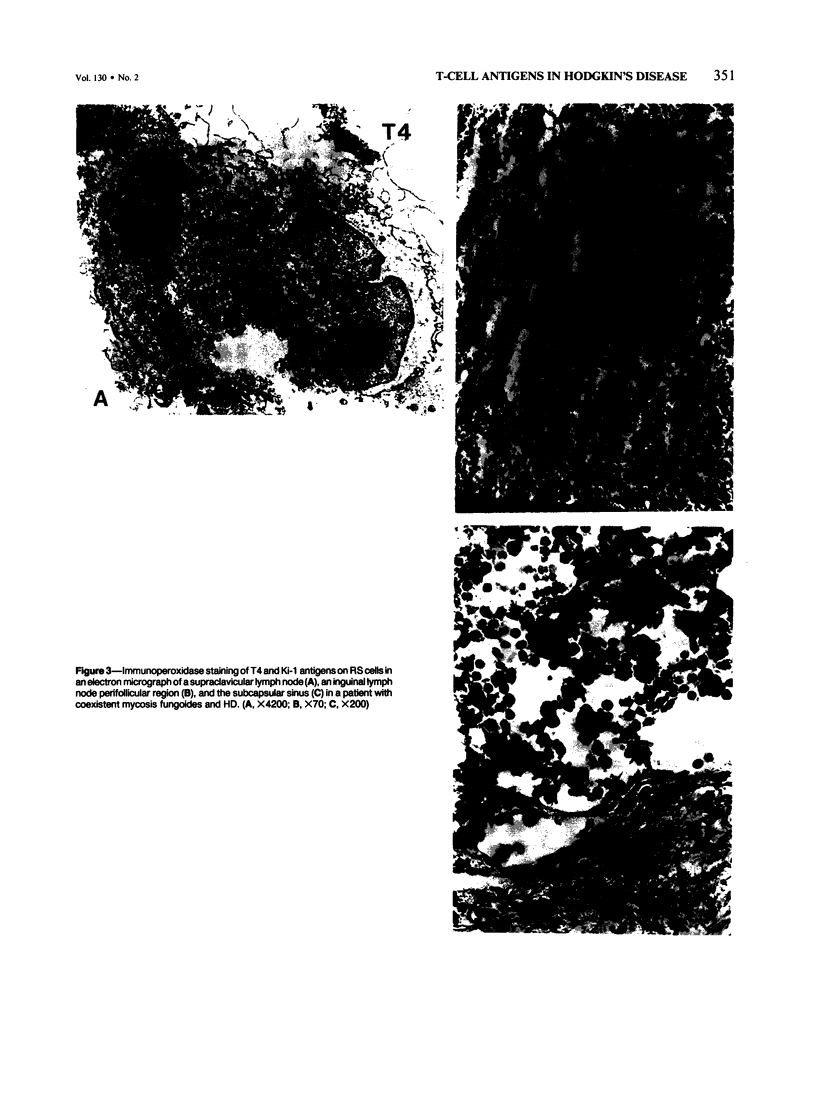
Expression of T-cell antigens by Reed-Sternberg (RS) cells has not been detected in most studies of Hodgkin's disease (HD). The authors employed an improved method of fixation (paraformaldehyde-lysine-periodate), which sharply defined cell borders and revealed T-cell antigens on RS cells in 8 of 30 (27%) cases of HD. Antigen-specific staining was confirmed by immunoelectron microscopy. RS cells expressed T11 (8/8 cases), Leu-3 or T4 (4/8 cases), Leu-4 or T3 (3/8 cases), but not other T-cell specific antigens (Leu-1, T8, T6, 3A1). RS cells were negative for leukocyte common antigen (LCA/T200), in contrast to positive LCA/T200 staining of RS-like cells in T-cell lymphomas. RS cells in all HD cases were positive for Ki-1 and/or Leu-M1 antigens. The percentage of RS cells expressing T-cell antigens was less than 20% (2 cases), 20-50% (3 cases), or greater than 50% (3 cases). This percentage and the specific T-cell antigens expressed varied in tissues from different sites in each of 2 cases. Expression of T-cell antigens by RS cells was found in nodular sclerosis (6 of 20 cases) and mixed cellularity (2 of 5 cases) but not in lymphocyte predominance (2 cases), lymphocyte depletion (1 case), or unclassified types (2 cases). Two cases of nodular sclerosis contained areas of necrosis surrounded by sheets of lacunar cells (syncytial variant of NSHD). Two other cases were associated with cutaneous lymphoma. One of these cases was mixed cellularity HD, which appeared to be confined to the skin. In a second case, tumor cells of similar phenotype (T4+, Ki-1+) were found in skin and lymph nodes of a patient with coexistent mycosis fungoides and HD. These results are consistent with an origin of RS cells from T cells in some cases of nodular sclerosing and mixed cellularity HD. They also suggest that the same cell type, an activated helper T-cell, is involved in the pathogenesis of both skin lesions and lymphadenopathy of some patients with coexistent mycosis fungoides and HD.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AISENBERG A. C. Studies on delayed hypersensitivity in Hodgkin's disease. J Clin Invest. 1962 Nov;41:1964–1970. doi: 10.1172/JCI104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulaziz Z., Mason D. Y., Stein H., Gatter K. C., Nash J. R. An immunohistological study of the cellular constituents of Hodgkin's disease using a monoclonal antibody panel. Histopathology. 1984 Jan;8(1):1–25. doi: 10.1111/j.1365-2559.1984.tb02318.x. [DOI] [PubMed] [Google Scholar]
- Braylan R. C., Jaffe E. S., Berard C. W. Letter: Surface characteristics of Hodgkin's lymphoma cells. Lancet. 1974 Nov 30;2(7892):1328–1329. doi: 10.1016/s0140-6736(74)90198-6. [DOI] [PubMed] [Google Scholar]
- Brinker M. G., Poppema S., Buys C. H., Timens W., Osinga J., Visser L. Clonal immunoglobulin gene rearrangements in tissues involved by Hodgkin's disease. Blood. 1987 Jul;70(1):186–191. [PubMed] [Google Scholar]
- Duque R. E., Lloyd R. V., Headington J. T., Schnitzer B. Site-dependent phenotypic heterogeneity in peripheral T-cell lymphoma. Hum Pathol. 1986 Sep;17(9):967–970. doi: 10.1016/s0046-8177(86)80649-9. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni M., Hofman F. M., Parker J. W., Lukes R. J., Taylor C. R. B- and T-lymphocytes in Hodgkin's disease. An immunohistochemical study utilizing heterologous and monoclonal antibodies. Cancer. 1985 Feb 15;55(4):728–737. doi: 10.1002/1097-0142(19850215)55:4<728::aid-cncr2820550409>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E. Common activated helper-T-cell origin for lymphomatoid papulosis, mycosis fungoides, and some types of Hodgkin's disease. Lancet. 1985 Oct 19;2(8460):864–865. doi: 10.1016/s0140-6736(85)90128-x. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Newcom S. R., Gold S. B., Stites D. P. Letter: Origin of Hodgkin's cell. Lancet. 1974 Jul 20;2(7873):167–168. doi: 10.1016/s0140-6736(74)91602-x. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. R., Castleman B. Hodgkin's disease of the thymus gland. Cancer. 1974 Jun;33(6):1615–1623. doi: 10.1002/1097-0142(197406)33:6<1615::aid-cncr2820330622>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J., Ye Y. L., Harrington D. S., Armitage J. O., Weisenburger D. D. Monoclonal antibodies marking T lymphocytes in paraffin-embedded tissue. Am J Pathol. 1987 Apr;127(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J. Criteria for involvement of lymph node, bone marrow, spleen, and liver in Hodgkin's disease. Cancer Res. 1971 Nov;31(11):1755–1767. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Nowinski R. C., Brown M. A. A new human T-cell differentiation antigen: unexpected expression on chronic lymphocytic leukemia cells. Immunogenetics. 1980;11(5):429–439. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Muramoto L. M., Kadin M. E. Improved detection of lymphoid cell surface antigens in tissues fixed in periodate-lysine-paraformaldehyde (PLP). Am J Clin Pathol. 1987 Nov;88(5):589–595. doi: 10.1093/ajcp/88.5.589. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Battifora H. A. Human homologue of murine T200 glycoprotein. J Exp Med. 1980 Oct 1;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Order S. E., Hellman S. Pathogenesis of Hodgkin's disease. Lancet. 1972 Mar 11;1(7750):571–573. doi: 10.1016/s0140-6736(72)90360-1. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Minowada J., Erikson J., Croce C. M., Rovera G. Lineage infidelity of a human myelogenous leukemia cell line. Blood. 1984 Nov;64(5):1059–1063. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Strickler J. G., Michie S. A., Warnke R. A., Dorfman R. F. The "syncytial variant" of nodular sclerosing Hodgkin's disease. Am J Surg Pathol. 1986 Jul;10(7):470–477. doi: 10.1097/00000478-198607000-00004. [DOI] [PubMed] [Google Scholar]
- Stuart A. E., Williams A. R., Habeshaw J. A. Rosetting and other reactions of the Reed-Sternberg cell. J Pathol. 1977 Jun;122(2):81–90. doi: 10.1002/path.1711220205. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Hu E., Warnke R. A., Sklar J. Immunoglobulin gene rearrangements in Hodgkin's disease. Hum Pathol. 1986 Oct;17(10):1009–1014. doi: 10.1016/s0046-8177(86)80084-3. [DOI] [PubMed] [Google Scholar]