Abstract
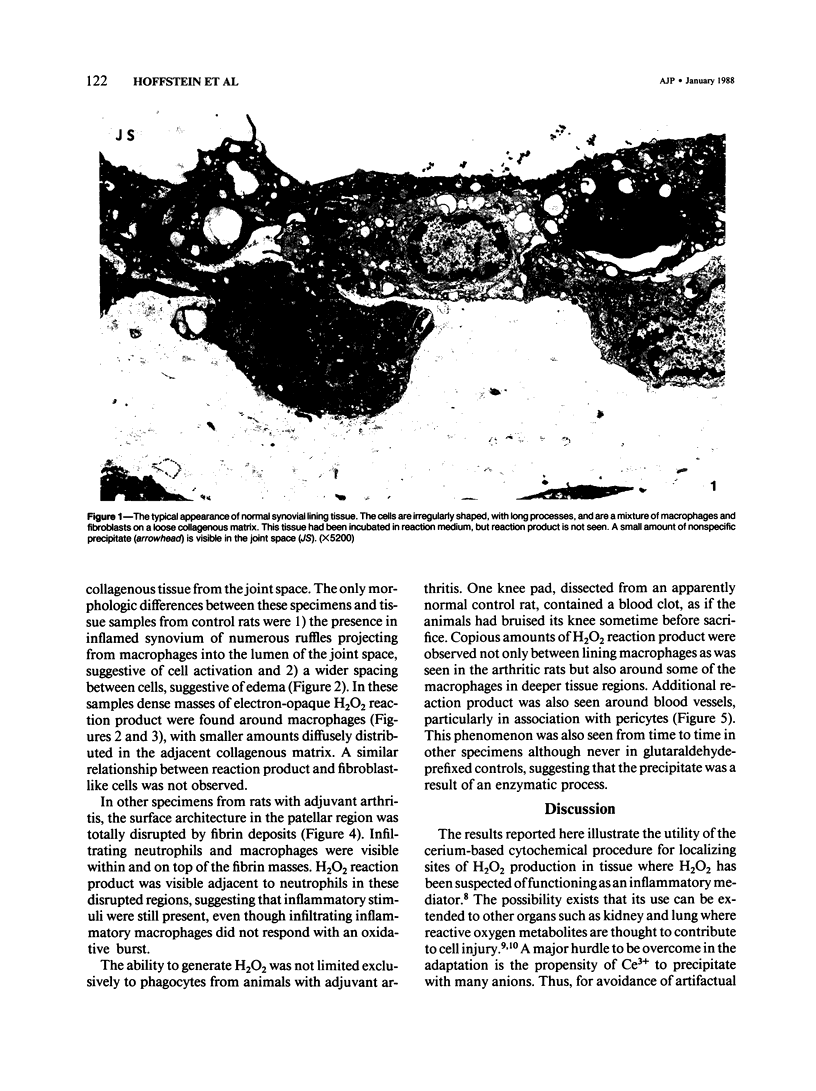
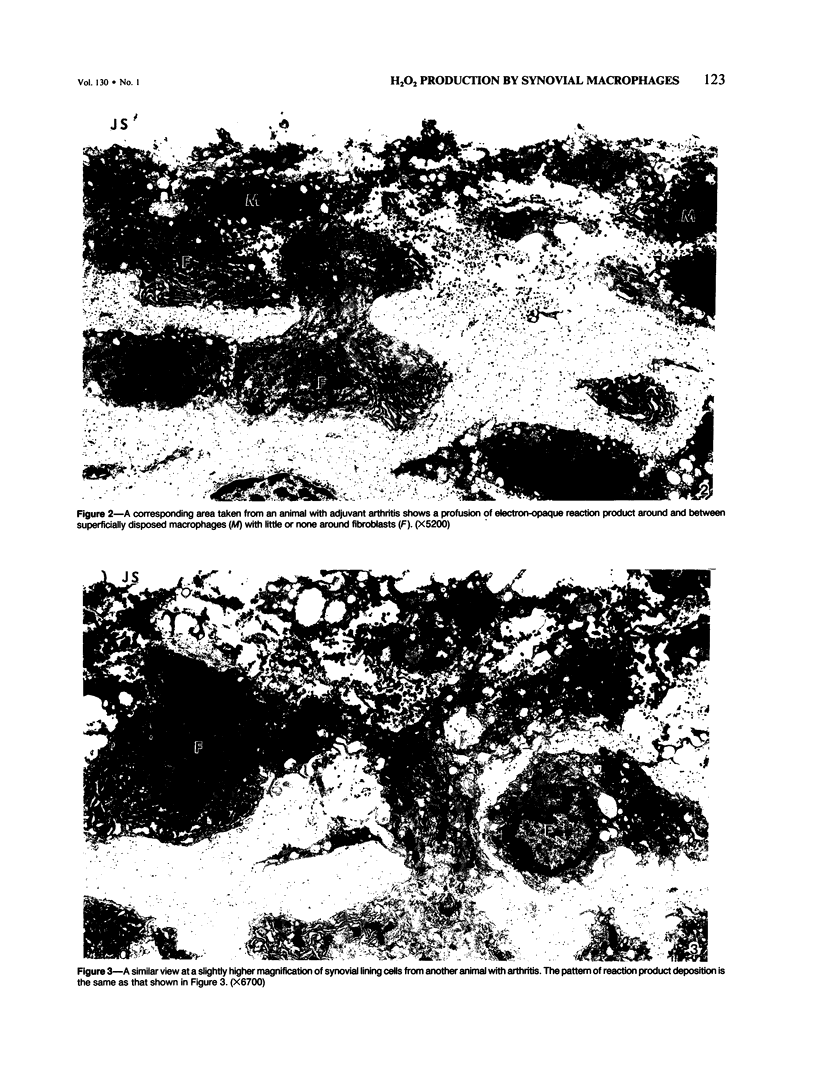
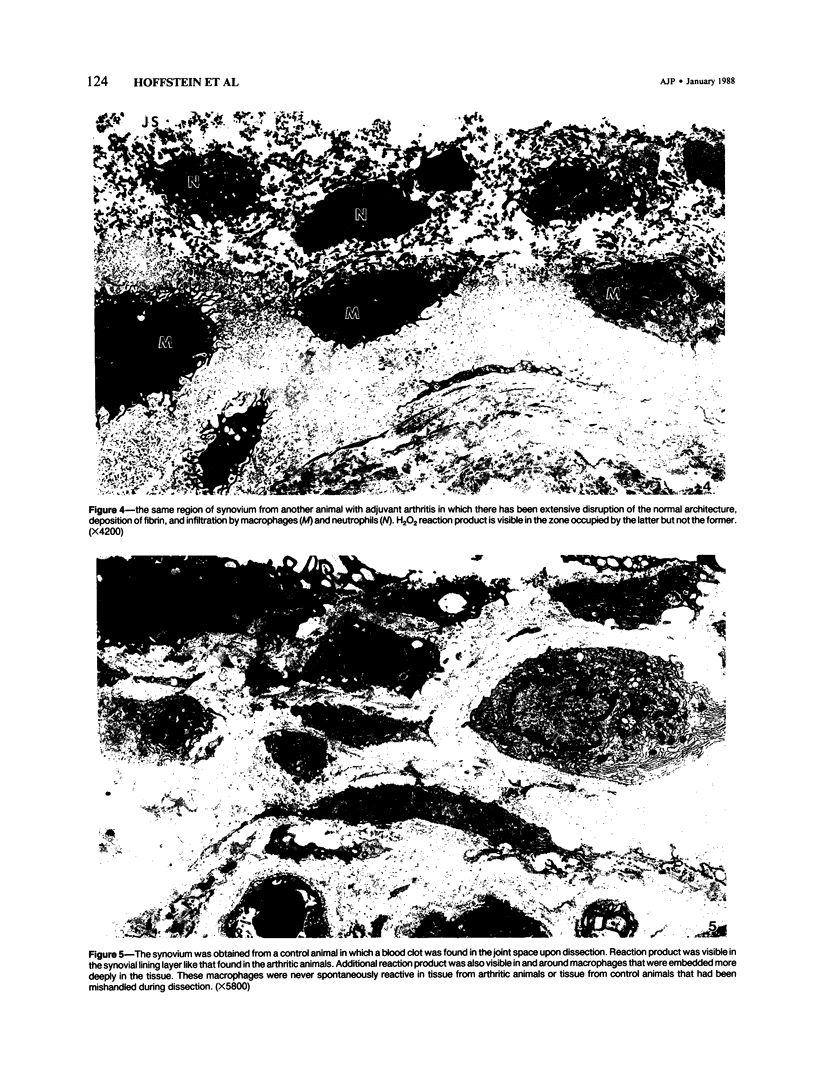
Generation of toxic oxygen metabolites by inflammatory cells is considered to be one of the mechanisms by which inflammation produces tissue injury. This concept is based on in vitro studies of purified leukocyte populations because it has not been possible to assess production of these metabolites in tissues. In order to determine whether or not inflammatory cells in tissue generate H2O2, the authors modified an earlier cytochemical method for the localization of H2O2. The incubation medium consists of 0.5 mM CeCl3 in a Hepes-buffered balanced salt solution with Cl- as the only anion. Synovial tissue from the knees of normal and 16-day adjuvant arthritic rats was incubated in this medium for 30 minutes and then fixed and processed for electron microscopy. No H2O2 reaction product was visible in normal synovium. In contrast, patchy deposits of H2O2 reaction product were seen adjacent to a subpopulation of synovial lining macrophages in synovium from inflamed knee joints. These data show that rat synovial macrophages are capable of generating H2O2 when appropriately stimulated and that such a stimulus is present in adjuvant arthritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., DiMartino M. J., Hanna N. Macrophage activation in rat models of inflammation and arthritis: determination of markers of stages of activation. Cell Immunol. 1986 Nov;103(1):54–64. doi: 10.1016/0008-8749(86)90067-5. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Sung C. P. Rat macrophage treatment with lipopolysaccharide leads to a reduction in respiratory burst product secretion and a decrease in NADPH oxidase affinity. Cell Immunol. 1987 Aug;108(1):109–119. doi: 10.1016/0008-8749(87)90197-3. [DOI] [PubMed] [Google Scholar]
- Rowley D., Gutteridge J. M., Blake D., Farr M., Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1984 Jun;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Till G. O., Hatherill J. R., Tourtellotte W. W., Lutz M. J., Ward P. A. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am J Pathol. 1985 Jun;119(3):376–384. [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Unanue E. R., Needleman P. Monocyte migration explains the changes in macrophage arachidonate metabolism during the immune response. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9655–9659. doi: 10.1073/pnas.83.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]







