Abstract
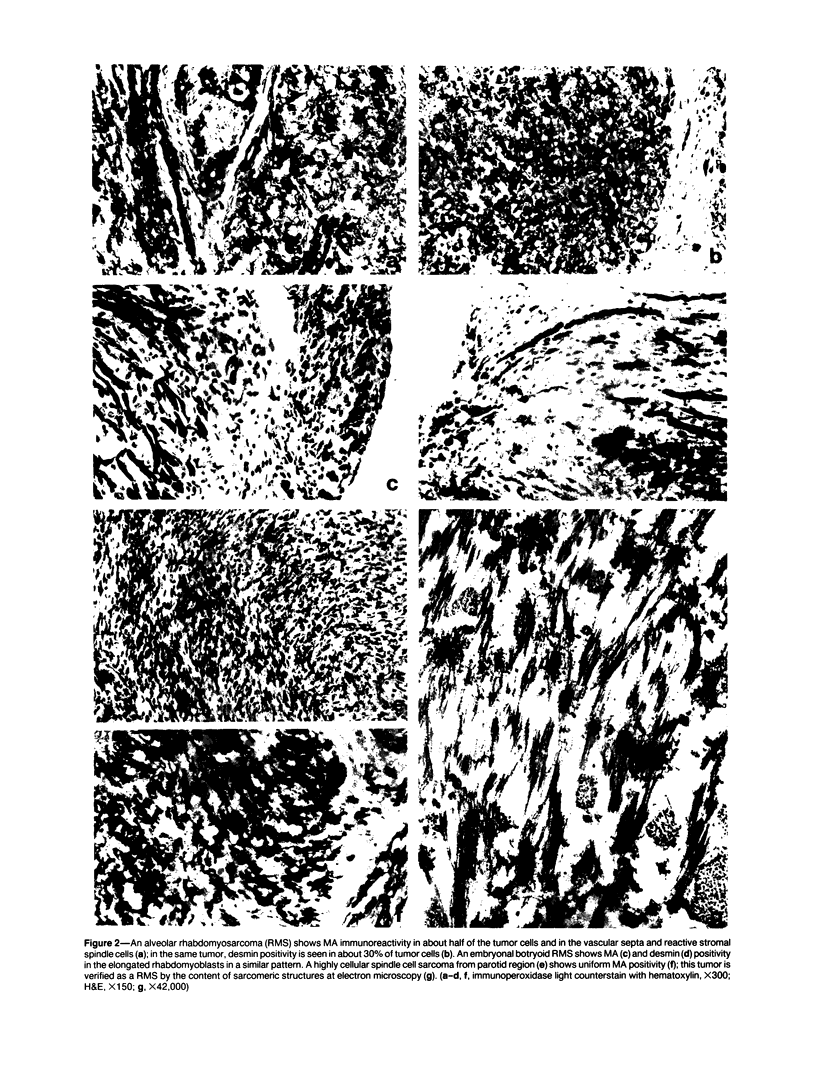
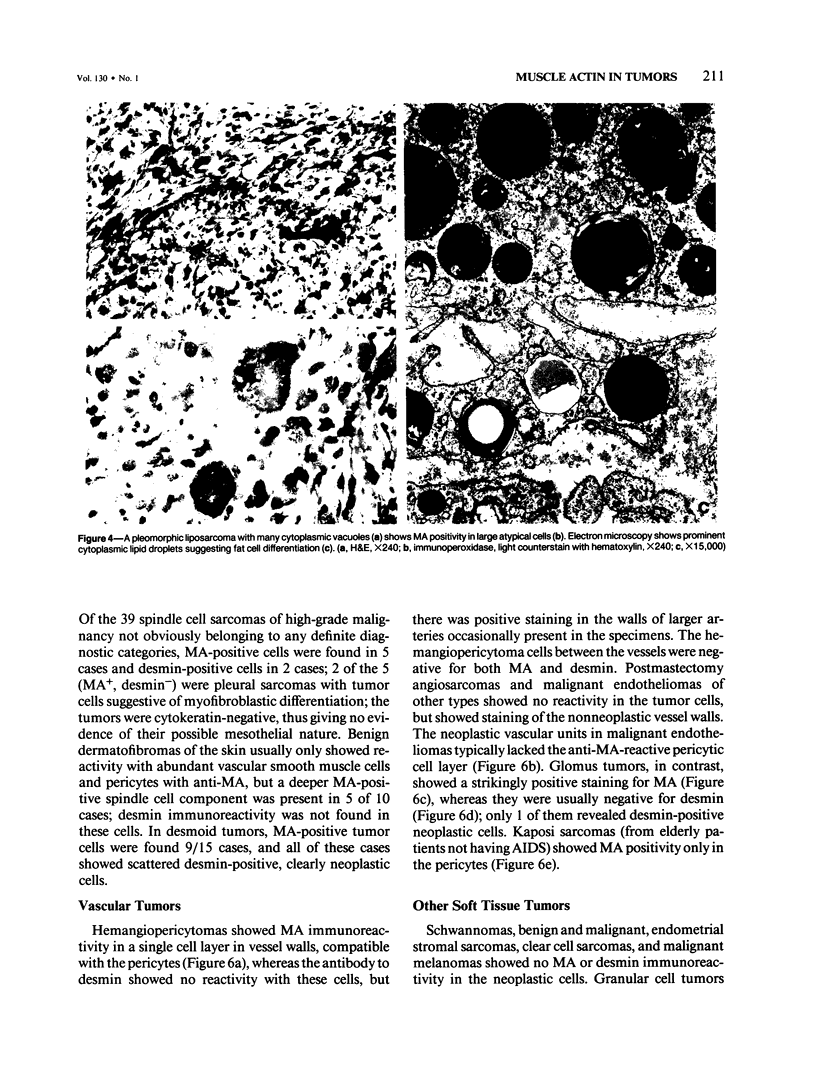
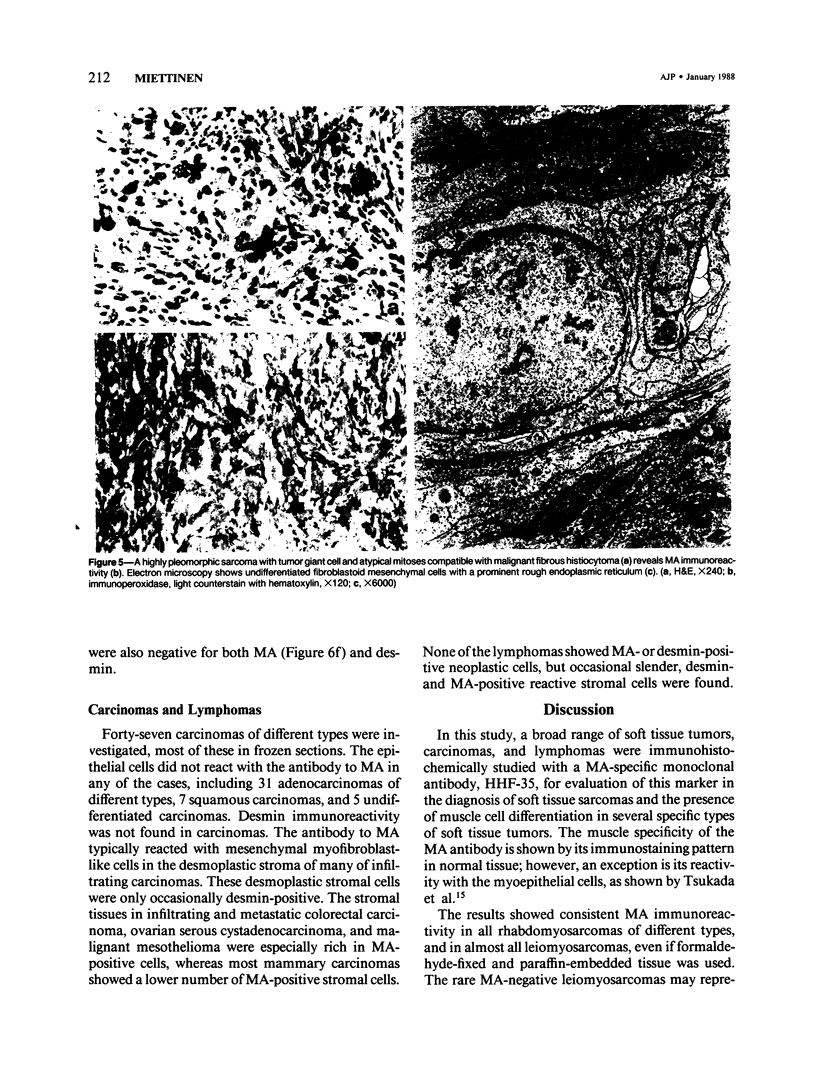
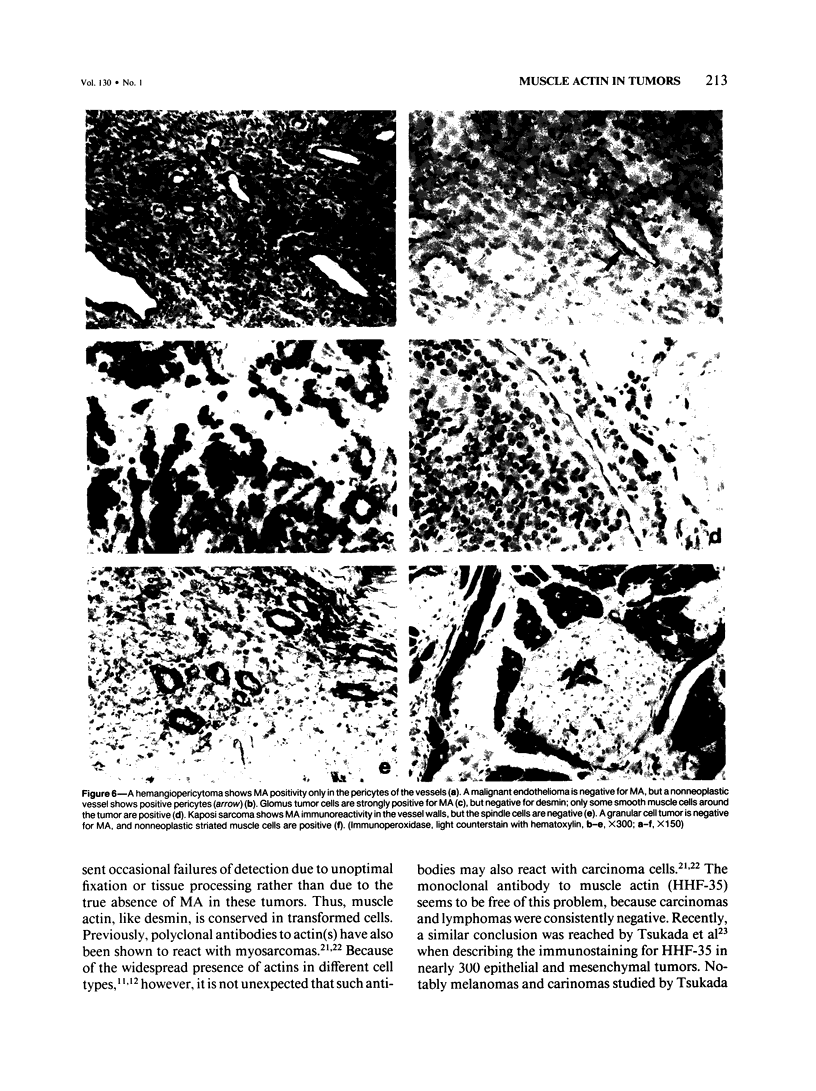
A series of soft tissue tumors, melanomas, carcinomas, and lymphomas were studied immunohistochemically for the presence of muscle actins (MA) with the monoclonal antibody HHF-35, and for the presence of desmin for comparison. In nonneoplastic tissues, MA immunoreactivity was present in skeletal and smooth muscle cells, in the pericytes of small vessels, and in the myoepithelial cells. Desmin immunoreactivity had a similar distribution, except that the pericytes of small vessels and myoepithelial cells were negative. All 17 rhabdomyosarcomas were positive for both MA and desmin. Of leiomyosarcomas, 31/32 were positive for MA, and 29/32 for desmin. In pleomorphic undifferentiated sarcomas (malignant fibrous histiocytomas) MA and desmin-positive cells were present in 9/35 and 5/35 cases, respectively. Three of five pleomorphic liposarcomas showed MA-positive tumor cells, which were also desmin-positive in one case. Desmoid tumors often showed a moderate number of both desmin- and MA-positive cells. Hemangiopericytoma, Kaposi's sarcoma, and endometrial stromal sarcoma showed MA-positive staining only in the pericytes and not in the neoplastic cells. In various types of carcinomas, melanomas, and lymphomas, MA- or desmin-positive neoplastic cells were not identified. MA, but not desmin, was present in the desmoplastic stroma in many carcinomas. Both MA and desmin are good markers for muscle differentiation and especially serve to identify rhabdomyosarcomas and leiomyosarcomas. These markers are also present in some sarcomas currently regarded as nonmuscle tumors. This may suggest that some of these tumors have differentiation properties related to true myosarcomas. The absence of muscle actin, a pericytic marker, in hemangiopericytoma does not confirm the concept of pericytic nature of this tumor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Treuner J., Hölscher A., Weber K., Shauer A. Diagnosis of human childhood rhabdomyosarcoma of antibodies to desmin, the structural protein of muscle specific intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(2):203–215. doi: 10.1007/BF02892848. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Weber K., Droste R., Osborn M. Desmin is a specific marker for rhabdomyosarcomas of human and rat origin. Am J Pathol. 1985 Jan;118(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Brooks J. J. The significance of double phenotypic patterns and markers in human sarcomas. A new model of mesenchymal differentiation. Am J Pathol. 1986 Oct;125(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Bussolati G., Alfani V., Weber K., Osborn M. Immunocytochemical detection of actin on fixed and embedded tissues: its potential use in routine pathology. J Histochem Cytochem. 1980 Feb;28(2):169–173. doi: 10.1177/28.2.6986431. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Alveolar rhabdomyosarcoma. Demonstration of the muscle type of intermediate filament protein, desmin, as a diagnostic aid. Am J Pathol. 1982 Aug;108(2):246–251. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Expression of intermediate filaments in soft-tissue sarcomas. Int J Cancer. 1982 Nov 15;30(5):541–546. doi: 10.1002/ijc.2910300502. [DOI] [PubMed] [Google Scholar]
- Molenaar W. M., Oosterhuis J. W., Oosterhuis A. M., Ramaekers F. C. Mesenchymal and muscle-specific intermediate filaments (vimentin and desmin) in relation to differentiation in childhood rhabdomyosarcomas. Hum Pathol. 1985 Aug;16(8):838–843. doi: 10.1016/s0046-8177(85)80256-2. [DOI] [PubMed] [Google Scholar]
- Mukai K., Schollmeyer J. V., Rosai J. Immunohistochemical localization of actin: applications in surgical pathology. Am J Surg Pathol. 1981 Jan;5(1):91–97. doi: 10.1097/00000478-198101000-00013. [DOI] [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Reddick R. L., Michelitch H., Triche T. J. Malignant soft tissue tumors (malignant fibrous histiocytoma, pleomorphic liposarcoma, and pleomorphic rhabdomyosarcoma): an electron microscopic study. Hum Pathol. 1979 May;10(3):327–343. doi: 10.1016/s0046-8177(79)80029-5. [DOI] [PubMed] [Google Scholar]
- Schürch W., Seemayer T. A., Lagacé R., Gabbiani G. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1984;403(4):323–336. doi: 10.1007/BF00737283. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol. 1979 Dec;3(6):525–533. doi: 10.1097/00000478-197912000-00005. [DOI] [PubMed] [Google Scholar]
- Stiller D., Katenkamp D. Cellular features in desmoid fibromatosis and well-differentiated fibrosarcomas: an electron microscopic study. Virchows Arch A Pathol Anat Histol. 1975 Dec 31;369(2):155–164. doi: 10.1007/BF00433241. [DOI] [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Tsuneyoshi M., Enjoji M., Shinohara N. Malignant fibrous histiocytoma. An electron microscopic study of 17 cases. Virchows Arch A Pathol Anat Histol. 1981;392(2):135–145. doi: 10.1007/BF00430816. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]