Abstract
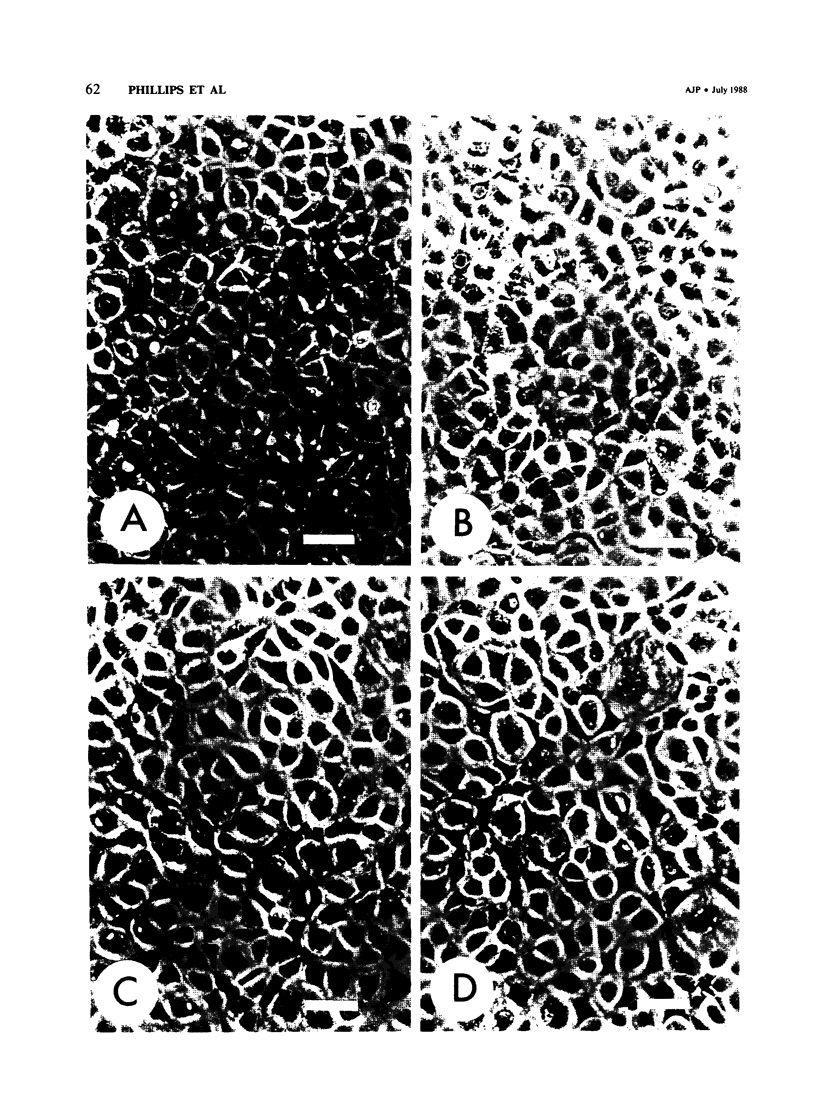
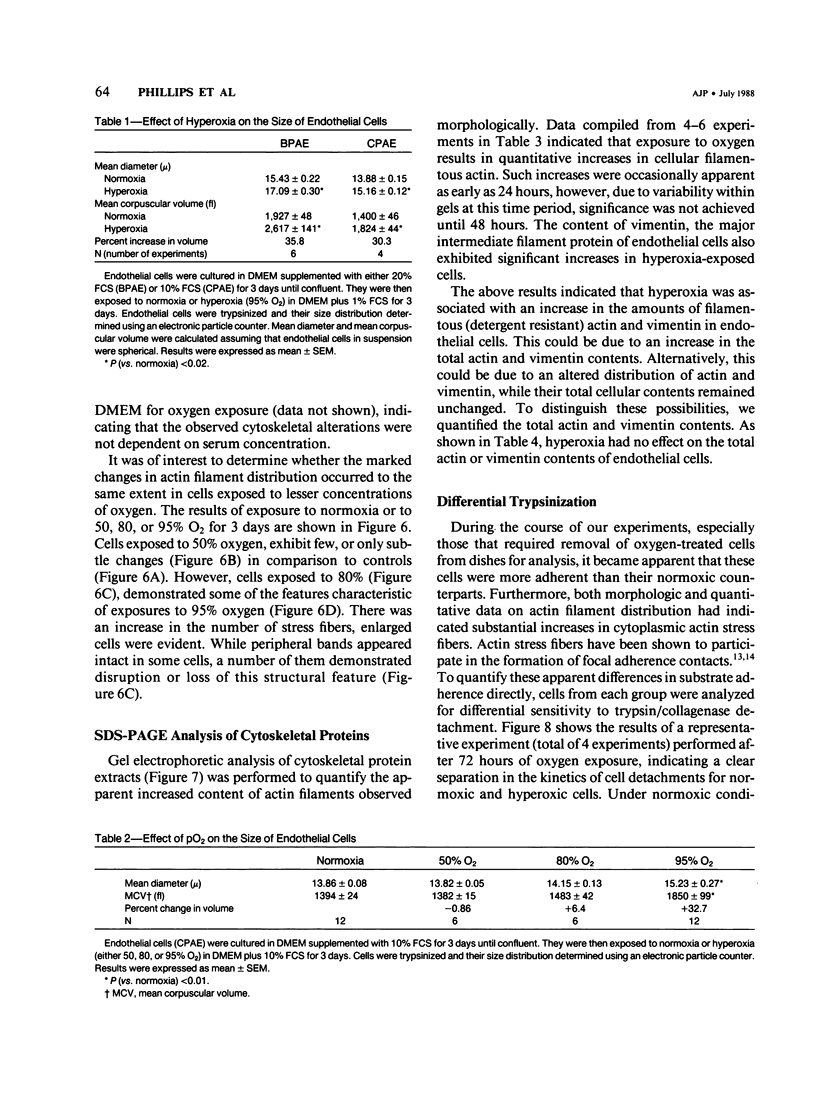
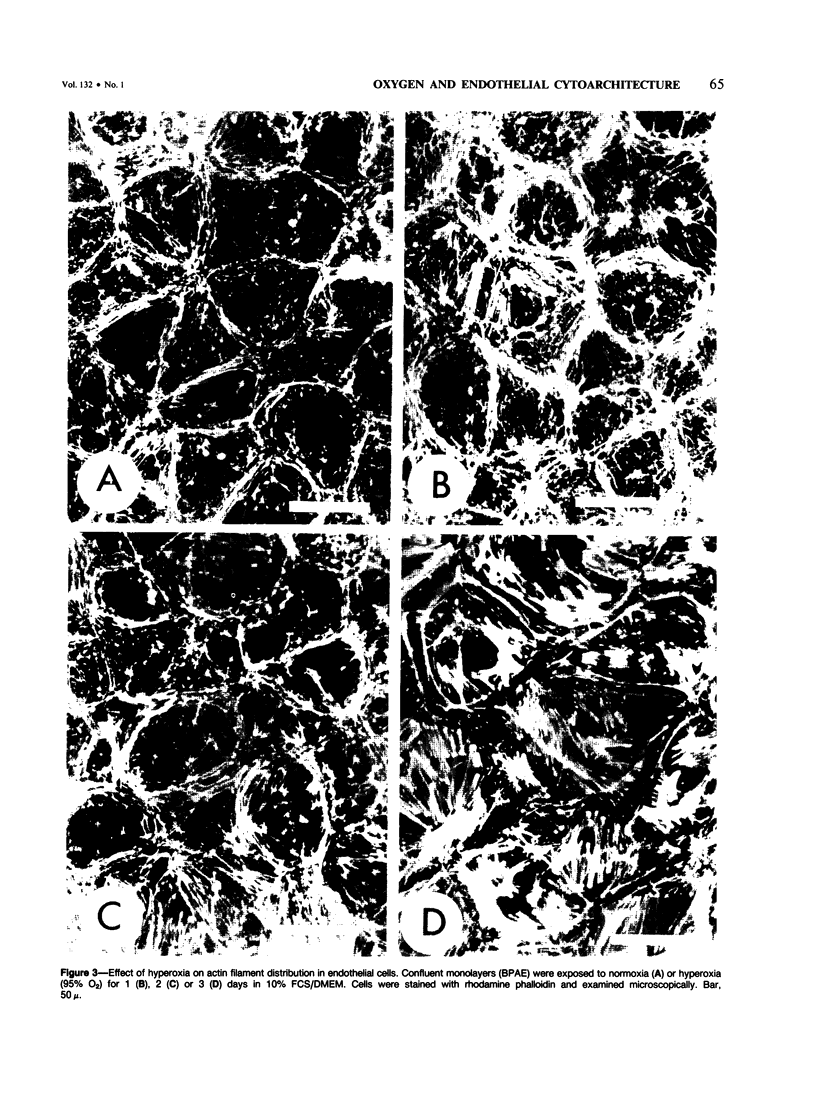
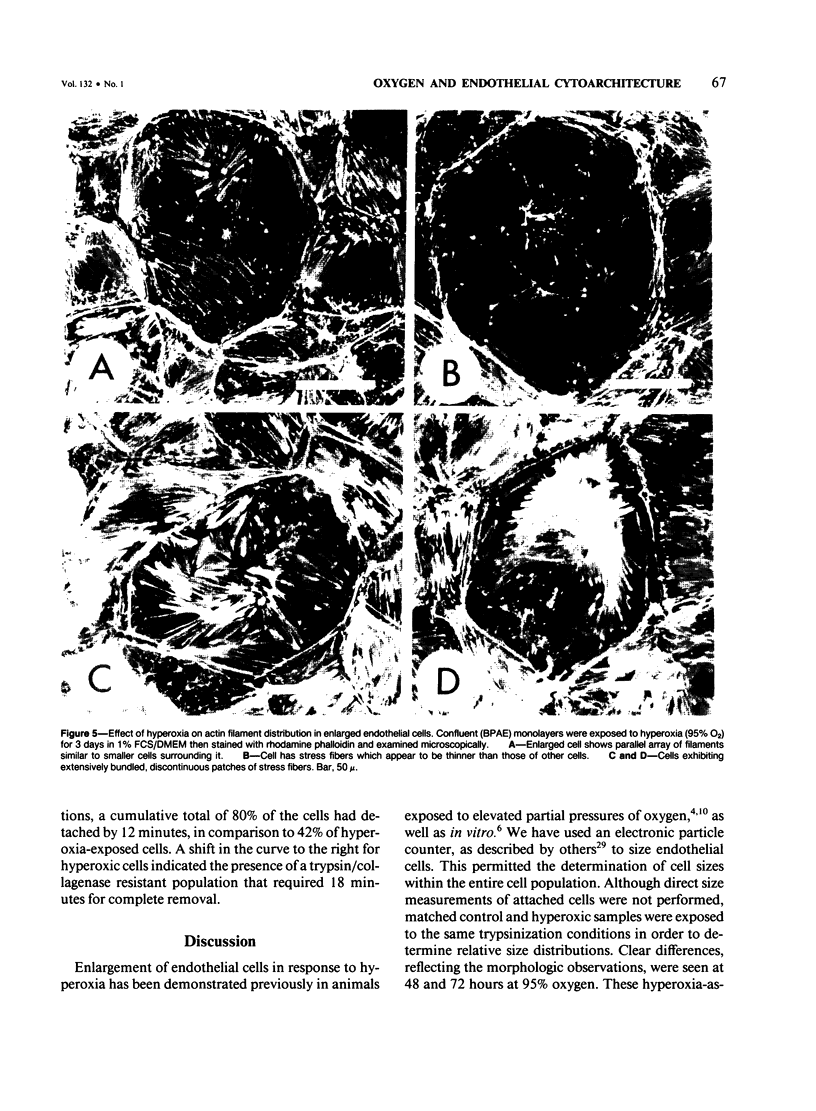
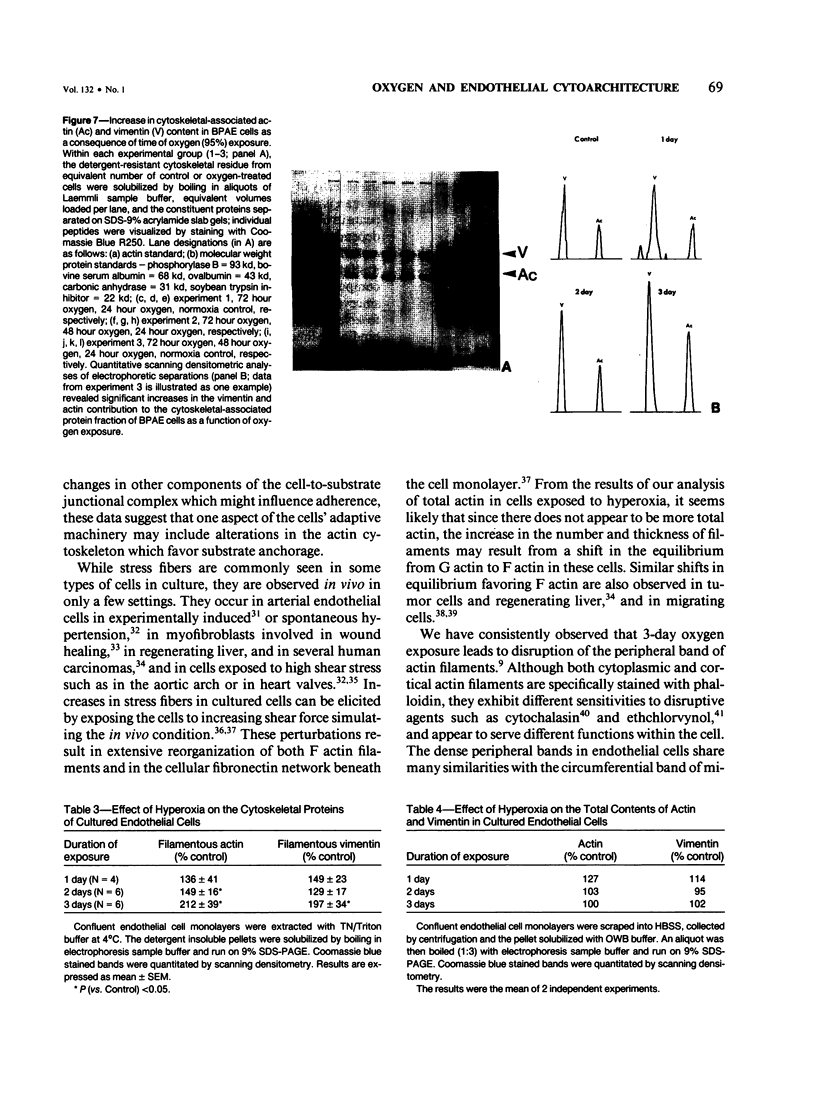
When confluent pulmonary artery endothelial cells in culture were exposed to hyperoxia (95% O2 and 5% CO2), they became enlarged and mean corpuscular volume increased 30-35%. Rhodamine-phalloidin staining of actin filaments demonstrated that hyperoxia was associated with a progressive alteration in the actin distribution. Three days after oxygen exposure, the number and thickness of cytoplasmic stress fibers were increased, while the peripheral bands were disrupted or absent. Sodium dodecyl sulfate polyacrylamide gel electrophoresis revealed that the amount of filamentous actin was increased in oxygen-exposed cells, while the total actin content remained unchanged, suggesting that oxygen exposure shifted the equilibrium from G actin to F actin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Burn P., Burger M. M. The cytoskeletal protein vinculin contains transformation-sensitive, covalently bound lipid. Science. 1987 Jan 23;235(4787):476–479. doi: 10.1126/science.3099391. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Frank L., Massaro D. Oxygen toxicity. Am J Med. 1980 Jul;69(1):117–126. doi: 10.1016/0002-9343(80)90509-4. [DOI] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Hüttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978 Mar;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Elemer G., Guelpa C., Vallotton M. B., Badonnel M. C., Hüttner I. Morphologic and functional changes of the aortic intima during experimental hypertension. Am J Pathol. 1979 Aug;96(2):399–422. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Lombardi D., Schwartz S. M. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–2364. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Schmid E., Franke W. W. Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation. 1983;23(3):189–205. doi: 10.1111/j.1432-0436.1982.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W., Wong M. K., Lacey C. In vitro reendothelialization. Microfilament bundle reorganization in migrating porcine endothelial cells. Arteriosclerosis. 1984 Mar-Apr;4(2):91–96. doi: 10.1161/01.atv.4.2.91. [DOI] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D., Wong A. J. Contractile proteins in endothelial cells. Ann N Y Acad Sci. 1982;401:50–60. doi: 10.1111/j.1749-6632.1982.tb25706.x. [DOI] [PubMed] [Google Scholar]
- Higgins P. J. Characterization of the growth inhibited substate induced in murine hepatic tumor cells during in vitro exposure to dimethylsulfoxide. Int J Cancer. 1986 Dec 15;38(6):889–899. doi: 10.1002/ijc.2910380617. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Jackson R. M. Pulmonary oxygen toxicity. Chest. 1985 Dec;88(6):900–905. doi: 10.1378/chest.88.6.900. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Douglas W. H., Deneke S. M., Fanburg B. L. Ultrastructural changes in bovine pulmonary artery endothelial cells exposed to 80% O2 in vitro. In Vitro. 1983 Sep;19(9):714–722. doi: 10.1007/BF02628963. [DOI] [PubMed] [Google Scholar]
- Lipkin M., Higgins P. Biological markers of cell proliferation and differentiation in human gastrointestinal diseases. Adv Cancer Res. 1988;50:1–24. doi: 10.1016/s0065-230x(08)60433-9. [DOI] [PubMed] [Google Scholar]
- Low R. B., Chaponnier C., Gabbiani G. Organization of actin in epithelial cells during regenerative and neoplastic conditions. Correlation of morphologic, immunofluorescent, and biochemical findings. Lab Invest. 1981 Apr;44(4):359–367. [PubMed] [Google Scholar]
- Madara J. L., Barenberg D., Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986 Jun;102(6):2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Meza I., Beaty G., Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin O., Patry P., Lafleur L. Heterogeneity of endothelial cells of adult rat liver as resolved by sedimentation velocity and flow cytometry. J Cell Physiol. 1984 Jun;119(3):327–334. doi: 10.1002/jcp.1041190311. [DOI] [PubMed] [Google Scholar]
- Opas M., Kalnins V. I. Spatial distribution of cortical proteins in cells of epithelial sheets. Cell Tissue Res. 1985;239(2):451–454. doi: 10.1007/BF00218027. [DOI] [PubMed] [Google Scholar]
- Phillips P. G., Tsan M. F. Direct staining and visualization of endothelial monolayers cultured on synthetic polycarbonate filters. J Histochem Cytochem. 1988 May;36(5):551–554. doi: 10.1177/36.5.3356897. [DOI] [PubMed] [Google Scholar]
- Phillips P. G., Tsan M. F. Hyperoxia causes increased albumin permeability of cultured endothelial monolayers. J Appl Physiol (1985) 1988 Mar;64(3):1196–1202. doi: 10.1152/jappl.1988.64.3.1196. [DOI] [PubMed] [Google Scholar]
- Pratt P. C., Vollmer R. T., Shelburne J. D., Crapo J. D. Pulmonary morphology in a multihospital collaborative extracorporeal membrane oxygenation project. I. Light microscopy. Am J Pathol. 1979 Apr;95(1):191–214. [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Discrimination between the nuclear lamin and intermediate filament (cytokeratin/vimentin) proteins of rat hepatic tumor cells by differential solubility and electrophoretic criteria. Int J Biochem. 1987;19(12):1187–1192. doi: 10.1016/0020-711x(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Greenburg G., Gospodarowicz D. Synthesis and distribution of cytoskeletal elements in endothelial cells as a function of cell growth and organization. J Cell Physiol. 1982 Feb;110(2):129–141. doi: 10.1002/jcp.1041100205. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Simon L. M. Lung cell oxidant injury. Enhancement of polymorphonuclear leukocyte-mediated cytotoxicity in lung cells exposed to sustained in vitro hyperoxia. J Clin Invest. 1982 Aug;70(2):342–350. doi: 10.1172/JCI110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Danis E. H., Del Vecchio P. J., Rosano C. L. Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun. 1985 Feb 28;127(1):270–276. doi: 10.1016/s0006-291x(85)80154-6. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Phillips P. G. L-2-oxothiazolidine-4-carboxylate protects cultured endothelial cells against hyperoxia-induced injury. Inflammation. 1988 Apr;12(2):113–121. doi: 10.1007/BF00916394. [DOI] [PubMed] [Google Scholar]
- Wechezak A. R., Viggers R. F., Sauvage L. R. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest. 1985 Dec;53(6):639–647. [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Cell-to-substratum contacts in living cells: a direct correlation between interference-reflexion and indirect-immunofluorescence microscopy using antibodies against actin and alpha-actinin. J Cell Sci. 1979 Jun;37:257–273. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Shasby D. M., Husted R. M. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985 Sep;76(3):1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H. R., Haschek W. M., Klein-Szanto A. J., Hakkinen P. J. Potentiation of diffuse lung damage by oxygen: determining variables. Am Rev Respir Dis. 1981 Jan;123(1):98–103. doi: 10.1164/arrd.1981.123.1.98. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986 Mar-Apr;6(2):212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D., Dahms T. Ethchlorvynol-induced pulmonary edema in rats. An ultrastructural study. Am J Pathol. 1984 Jun;115(3):447–457. [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D. The effect of ethchlorvynol on cultured endothelial cells. A model for the study of the mechanism of increased vascular permeability. Am J Pathol. 1985 Jun;119(3):505–512. [PMC free article] [PubMed] [Google Scholar]