Abstract
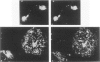
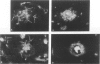
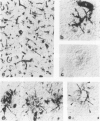
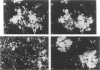
The senile plaque is one of the histopathologic changes that characterizes Alzheimer's disease and the aging brain. The histopathology of senile plaques was studied using double-labeling immunohistochemistry and lectin histochemistry with thioflavin S fluorescent microscopy in 9 cases of Alzheimer's disease, 2 nondemented elderly individuals, and 3 individuals with non-Alzheimer primary degenerative dementias. Every plaque that was visualized with thioflavin also had amyloid, but not all thioflavin-positive plaques contained neurites that could be recognized with specific monoclonal antibodies to paired helical filament, tau, or neurofilament epitopes. Some neurofilament-positive neurites were not visualized with thioflavin, but almost all tau-positive neurites were colabeled with thioflavin. Microglia were associated with most plaques. Most plaques were also surrounded by fibrous astrocytes. These results suggest that amyloid may be the common feature that defines senile plaques, but that other elements may be more specific for Alzheimer's disease, because extensive neuritic degeneration was seen only in Alzheimer brains and not in either nondemented elderly individuals with senile plaques or in non-Alzheimer dementia cases.
Full text
PDF


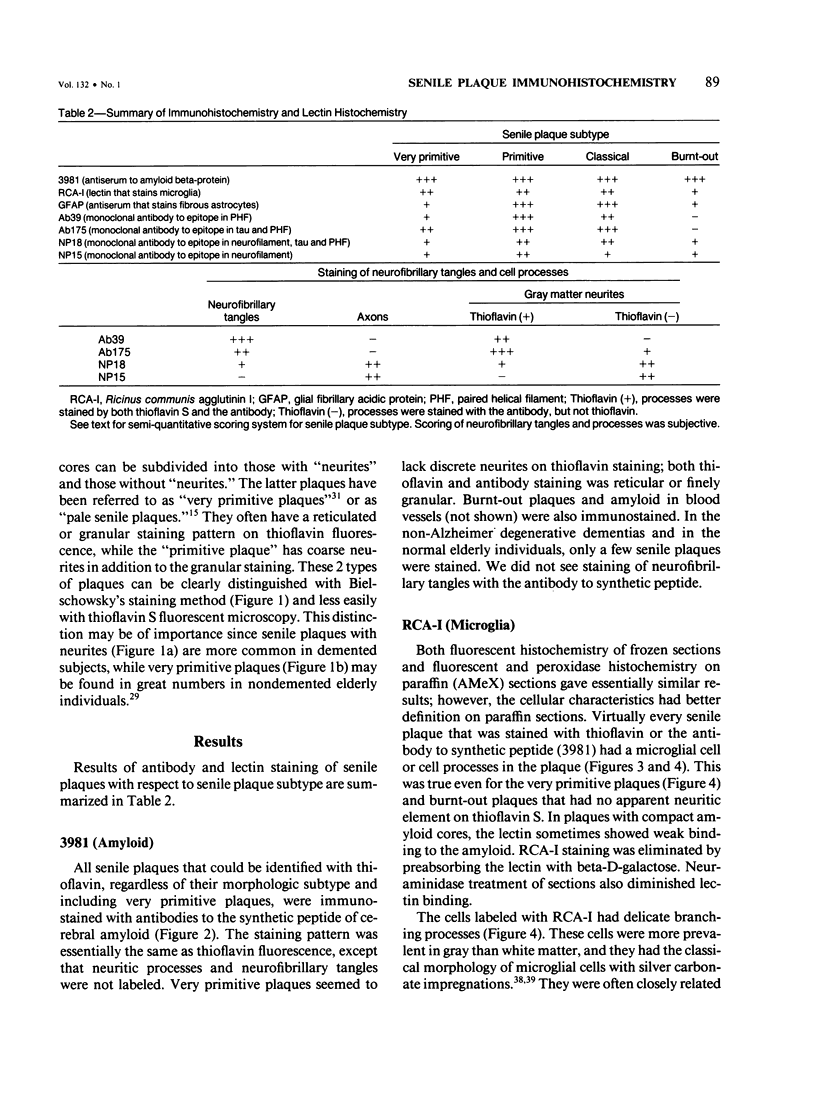

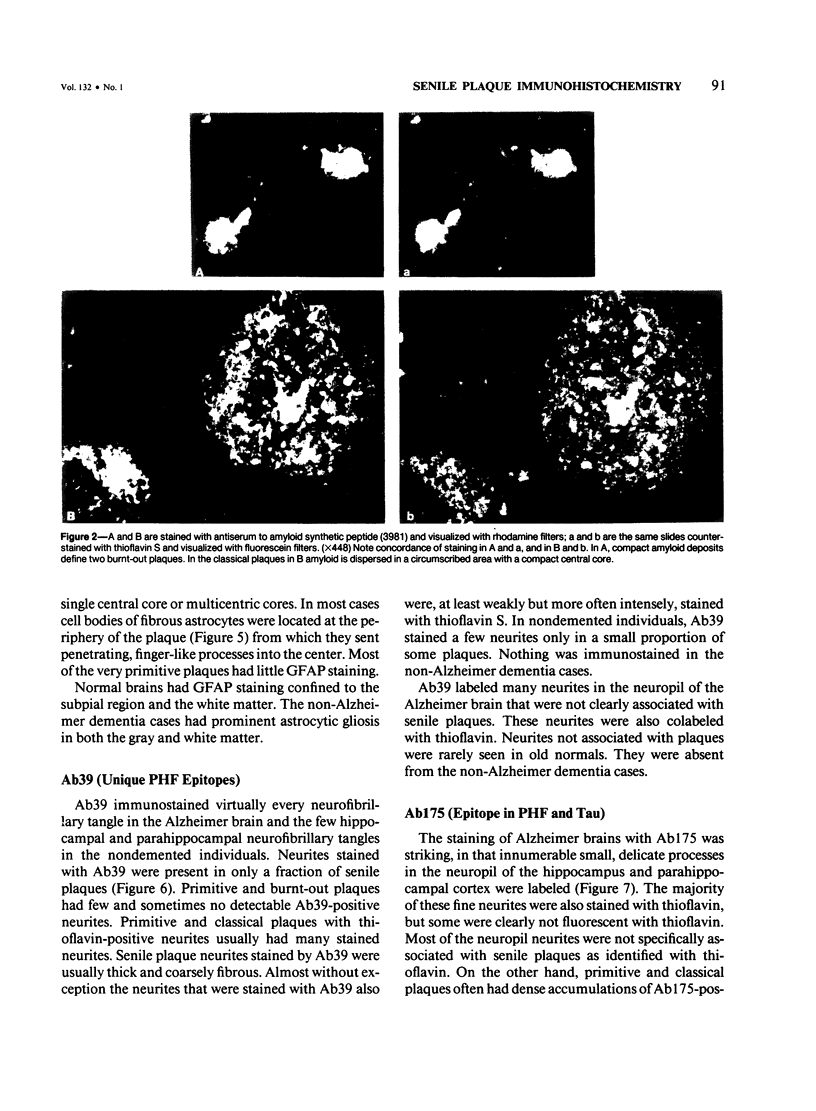




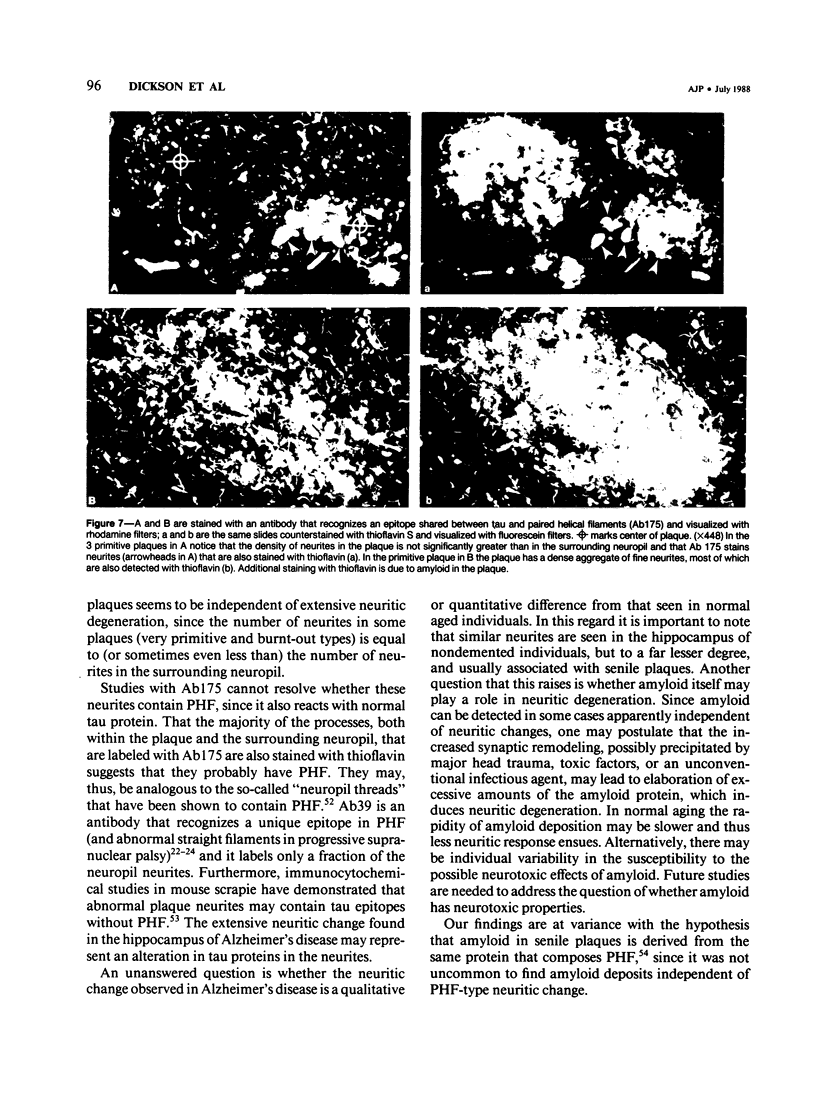





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Landon M., Kidd M., Lowe J. S., Reynolds G. P., Gardner A. Monoclonal antibodies raised against a subsequence of senile plaque core protein react with plaque cores, plaque periphery and cerebrovascular amyloid in Alzheimer's disease. Neurosci Lett. 1986 Jul 24;68(2):252–256. doi: 10.1016/0304-3940(86)90152-7. [DOI] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Bondareff W. Synaptic atrophy in the senescent hippocampus. Mech Ageing Dev. 1979 Jan;9(1-2):163–171. doi: 10.1016/0047-6374(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Fraser H., Flament-Durand J., Dickinson A. G. Amyloid scrapie plaques in mice, and Alzheimer senile plaques, share common antigens with tau, a microtubule-associated protein. Neurosci Lett. 1987 Jul 9;78(1):113–118. doi: 10.1016/0304-3940(87)90571-4. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Kress Y., Crowe A., Yen S. H. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 2. Demonstration of a common antigenic determinant between ANT and neurofibrillary degeneration in progressive supranuclear palsy. Am J Pathol. 1985 Aug;120(2):292–303. [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W. Multinucleated giant cells in acquired immunodeficiency syndrome encephalopathy. Origin from endogenous microglia? Arch Pathol Lab Med. 1986 Oct;110(10):967–968. [PubMed] [Google Scholar]
- Dickson D. W., Yen S. H., Horoupian D. S. Pick body-like inclusions in the dentate fascia of the hippocampus in Alzheimer's disease. Acta Neuropathol. 1986;71(1-2):38–45. doi: 10.1007/BF00687960. [DOI] [PubMed] [Google Scholar]
- Fuld P. A., Dickson D., Crystal H., Aronson M. K. Primitive plaques and memory dysfunction in normal and impaired elderly persons. N Engl J Med. 1987 Mar 19;316(12):756–756. doi: 10.1056/NEJM198703193161219. [DOI] [PubMed] [Google Scholar]
- Gibson P. H. Form and distribution of senile plaques seen in silver impregnated sections in the brains of intellectually normal elderly people and people with Alzheimer-type dementia. Neuropathol Appl Neurobiol. 1983 Sep-Oct;9(5):379–389. doi: 10.1111/j.1365-2990.1983.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Gibson P. H. Relationship between numbers of cortical argentophilic and congophilic senile plaques in the brains of elderly people with and without senile dementia of Alzheimer type. Gerontology. 1985;31(5):321–324. doi: 10.1159/000212716. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Anderson W., Evangelista I. The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol. 1967 Jan;26(1):25–39. doi: 10.1097/00005072-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita K., Tateishi J., Nagara H. Morphogenesis of amyloid plaques in mice with Creutzfeldt-Jakob disease. Acta Neuropathol. 1985;68(2):138–144. doi: 10.1007/BF00688635. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Wolozin B. L., Davies P., Kromer L. J., Damasio A. R. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 1988 Apr;23(4):371–379. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- KIDD M. ALZHEIMER'S DISEASE--AN ELECTRON MICROSCOPICAL STUDY. Brain. 1964 Jun;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Two monoclonal antibodies recognize Alzheimer's neurofibrillary tangles, neurofilament, and microtubule-associated proteins. J Neurochem. 1987 Feb;48(2):455–462. doi: 10.1111/j.1471-4159.1987.tb04114.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Campbell M. J., Terry R. D., Morrison J. H. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987 Jun;7(6):1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A., Brunnschweiler H., Lautenschlager C., Ulrich J. A special type of senile plaque, possibly an initial stage. Acta Neuropathol. 1987;74(2):133–141. doi: 10.1007/BF00692843. [DOI] [PubMed] [Google Scholar]
- Rogers J., Morrison J. H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci. 1985 Oct;5(10):2801–2808. doi: 10.1523/JNEUROSCI.05-10-02801.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudelli R. D., Ambler M. W., Wisniewski H. M. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol. 1984;64(4):273–281. doi: 10.1007/BF00690393. [DOI] [PubMed] [Google Scholar]
- Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986 Dec;125(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Schaumburg H. H., Spencer P. S. Recognizing neurotoxic disease. Neurology. 1987 Feb;37(2):276–278. doi: 10.1212/wnl.37.2.276. [DOI] [PubMed] [Google Scholar]
- Schechter R., Yen S. H., Terry R. D. Fibrous Astrocytes in senile dementia of the Alzheimer type. J Neuropathol Exp Neurol. 1981 Mar;40(2):95–101. doi: 10.1097/00005072-198103000-00002. [DOI] [PubMed] [Google Scholar]
- Scheibel A. B. The hippocampus: organizational patterns in health and senescence. Mech Ageing Dev. 1979 Jan;9(1-2):89–102. doi: 10.1016/0047-6374(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Willmer J. P., Kisilevsky R. Sulfated glycosaminoglycans in Alzheimer's disease. Hum Pathol. 1987 May;18(5):506–510. doi: 10.1016/s0046-8177(87)80036-9. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Tanzi R. E., Polinsky R. J., Haines J. L., Nee L., Watkins P. C., Myers R. H., Feldman R. G., Pollen D., Drachman D. The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science. 1987 Feb 20;235(4791):885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- Stam F. C., Wigboldus J. M., Smeulders A. W. Age incidence of senile brain amyloidosis. Pathol Res Pract. 1986 Oct;181(5):558–562. doi: 10.1016/S0344-0338(86)80149-2. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Hansen L. A., DeTeresa R., Davies P., Tobias H., Katzman R. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol. 1987 May;46(3):262–268. doi: 10.1097/00005072-198705000-00003. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 Sep-Oct;7(2):331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985 Mar;17(3):273–277. doi: 10.1002/ana.410170309. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Moretz R. C., Lossinsky A. S. Evidence for induction of localized amyloid deposits and neuritic plaques by an infectious agent. Ann Neurol. 1981 Dec;10(6):517–522. doi: 10.1002/ana.410100605. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Ghetti B., Terry R. D. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973 Oct;32(4):566–584. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H., Johnson A. B., Raine C. S., Kay W. J., Terry R. D. Senile plaques and cerebral amyloidosis in aged dogs. A histochemical and ultrastructural study. Lab Invest. 1970 Sep;23(3):287–296. [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Crowe A., Dickson D. W. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 1. Identification of polypeptides. Am J Pathol. 1985 Aug;120(2):282–291. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Dickson D. W., Crowe A., Butler M., Shelanski M. L. Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol. 1987 Jan;126(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- van Duinen S. G., Castaño E. M., Prelli F., Bots G. T., Luyendijk W., Frangione B. Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer disease. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5991–5994. doi: 10.1073/pnas.84.16.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]