Abstract
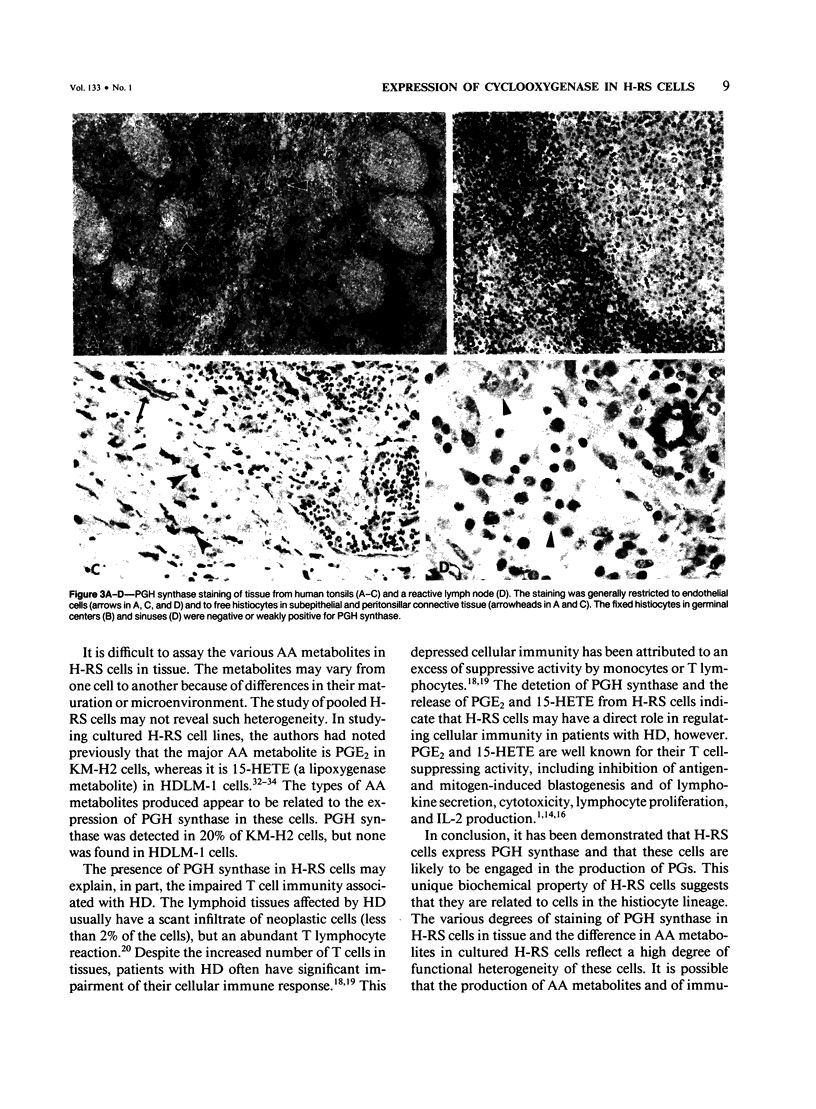
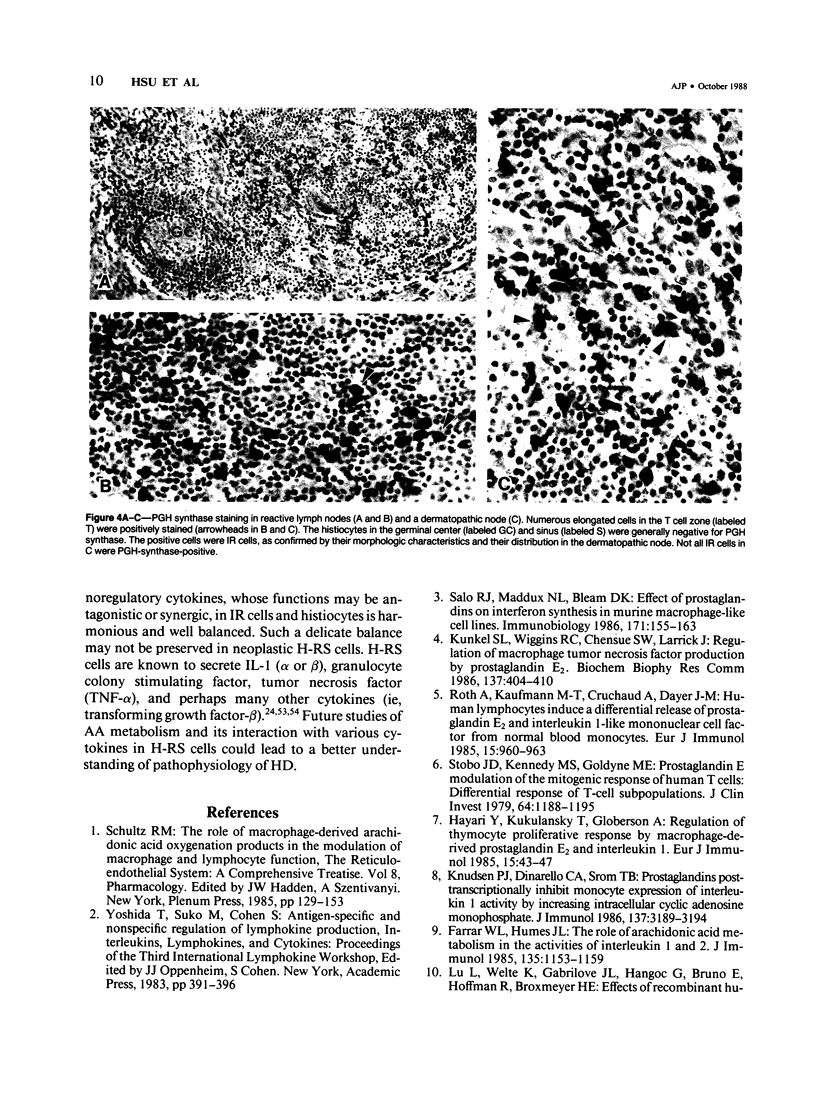
The synthesis of prostaglandin, prostacyclin, and thromboxane, which requires the enzyme prostaglandin H (PGH) synthase (cyclooxygenase), is a general property of histiocytes, monocytes, and Langerhans cells. Previously the authors reported the production of prostaglandin E2 in a Hodgkin's cell line, KM-H2, and suggested that these cells therefore have a functional similarity to histiocyte-related cells. The present study confirms that the Hodgkin's neoplastic (Reed-Sternberg, H-RS) cells in tissue are also capable of producing prostaglandins by demonstrating the presence of PGH synthase in these cells in B5-fixed, paraffin-embedded tissue sections. It was found that the H-RS cells from seven of ten patients with Hodgkin's disease were stained variously with anti-PGH synthase antibodies. In normal and reactive lymphoid tissues, anti-PGH synthase staining was restricted to histiocytes, endothelial cells, and interdigitating reticulum cells. Thus, this study provides further evidence for a possible relationship between H-RS cells and histiocytes or interdigitating reticulum cells; this relationship has also been supported by information obtained in extensive immunologic, biochemical, and cell-differentiation studies. The secretion of PGH-synthase products, especially prostaglandin E2, in H-RS cells may play a major role in the regulation of cellular immunity in patients with Hodgkin's disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenzana-Seisdedos F., Barbey S., Virelizier J. L., Kornprobst M., Nezelof C. Histiocytosis X. Purified (T6+) cells from bone granuloma produce interleukin 1 and prostaglandin E2 in culture. J Clin Invest. 1986 Jan;77(1):326–329. doi: 10.1172/JCI112296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Bryant R. W., Low C. E., Pupillo M. B., Vanderhoek J. Y. Regulation of T-lymphocyte mitogenesis by the leukocyte product 15-hydroxy-eicosatetraenoic acid (15-HETE). Cell Immunol. 1982 Feb;67(1):112–120. doi: 10.1016/0008-8749(82)90203-9. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Ribolini A. Interferon blocks interleukin 1-induced prostaglandin release from human peripheral monocytes. J Immunol. 1987 May 1;138(9):2857–2863. [PubMed] [Google Scholar]
- Burrichter H., Heit W., Schaadt M., Kirchner H., Diehl V. Production of colony-stimulating factors by Hodgkin cell lines. Int J Cancer. 1983 Mar 15;31(3):269–274. doi: 10.1002/ijc.2910310303. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D. L., Day J. S., Gauger J. A., Smith W. L. Monoclonal antibodies against PGH synthase: an immunoradiometric assay for quantitating the enzyme. Methods Enzymol. 1982;86:229–240. doi: 10.1016/0076-6879(82)86194-6. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Marnoy S. O., Rosenwasser L. J. Role of arachidonate metabolism in the immunoregulatory function of human leukocytic pyrogen/lymphocyte-activating factor/interleukin 1. J Immunol. 1983 Feb;130(2):890–895. [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Lok M. S., Diehl V., Minowada J. Hodgkin's disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10(5):487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Humes J. L. The role of arachidonic acid metabolism in the activities of interleukin 1 and 2. J Immunol. 1985 Aug;135(2):1153–1159. [PubMed] [Google Scholar]
- Fisher R. I., Bates S. E., Bostick-Bruton F., Tuteja N., Diehl V. Neoplastic cells obtained from Hodgkin's disease function as accessory cells for mitogen-induced human T cell proliferative responses. J Immunol. 1984 May;132(5):2672–2677. [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Fisher R. I. Implications of persistent T cell abnormalities for the etiology of Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):681–687. [PubMed] [Google Scholar]
- Hayari Y., Kukulansky T., Globerson A. Regulation of thymocyte proliferative response by macrophage-derived prostaglandin E2 and interleukin 1. Eur J Immunol. 1985 Jan;15(1):43–47. doi: 10.1002/eji.1830150109. [DOI] [PubMed] [Google Scholar]
- Hemler M., Lands W. E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976 Sep 25;251(18):5575–5579. [PubMed] [Google Scholar]
- Hillinger S. M., Herzig G. P. Impaired cell-mediated immunity in Hodgkin's disease mediated by suppressor lymphocytes and monocytes. J Clin Invest. 1978 Jun;61(6):1620–1627. doi: 10.1172/JCI109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Ho Y. S., Li P. J., Monheit J., Ree H. J., Sheibani K., Winberg C. D. L&H variants of Reed-Sternberg cells express sialylated Leu M1 antigen. Am J Pathol. 1986 Feb;122(2):199–203. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Phenotypes and phorbol ester-induced differentiation of human histiocytic lymphoma cell lines (U-937 and SU-DHL-1) and Reed-Sternberg cells. Am J Pathol. 1986 Feb;122(2):223–230. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Huang L. C., Hsu P. L., Ge Z. H., Ho Y. S., Cuttita F., Mulshine J. Biochemical and ultrastructural study of Leu M1 antigen in Reed-Sternberg cells: comparison with granulocytes and interdigitating reticulum cells. J Natl Cancer Inst. 1986 Aug;77(2):363–370. [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Pescovitz M. D., Hsu P. L. Monoclonal antibodies against SU-DHL-1 cells stain the neoplastic cells in true histiocytic lymphoma, malignant histiocytosis, and Hodgkin's disease. Blood. 1986 Jul;68(1):213–219. [PubMed] [Google Scholar]
- Hsu S. M. Phenotypic expression of cells of stationary elements in human lymphoid tissues. A histochemical and immunohistochemical study. Hematol Pathol. 1987;1(1):45–56. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Chakraborty S., Liu Y. F., Whang-Peng J., Lok M. S., Fukuhara S. Reed-Sternberg cells in Hodgkin's cell lines HDLM, L-428, and KM-H2 are not actively replicating: lack of bromodeoxyuridine uptake by multinuclear cells in culture. Blood. 1988 May;71(5):1382–1389. [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Hsu P. L., Lok M. S. Extracellular matrix does not induce the proliferation, but promotes the differentiation, of Hodgkin's cell line HDLM-1. Am J Pathol. 1987 Apr;127(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. The H-RS-like cells in infectious mononucleosis are transformed interdigitating reticulum cells. Am J Pathol. 1987 May;127(2):403–408. [PMC free article] [PubMed] [Google Scholar]
- Kamesaki H., Fukuhara S., Tatsumi E., Uchino H., Yamabe H., Miwa H., Shirakawa S., Hatanaka M., Honjo T. Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin's disease. Blood. 1986 Jul;68(1):285–292. [PubMed] [Google Scholar]
- Khansari N., Chou Y. K., Fudenberg H. H. Human monocyte heterogeneity: interleukin 1 and prostaglandin E2 production by separate subsets. Eur J Immunol. 1985 Jan;15(1):48–51. doi: 10.1002/eji.1830150110. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Kunkel S. L., Wiggins R. C., Chensue S. W., Larrick J. Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochem Biophys Res Commun. 1986 May 29;137(1):404–410. doi: 10.1016/0006-291x(86)91224-6. [DOI] [PubMed] [Google Scholar]
- McGuire J. C., Richard K. A., Sun F. F., Tracey D. E. Production of prostaglandin D2 by murine macrophage cell lines. Prostaglandins. 1985 Dec;30(6):949–967. doi: 10.1016/0090-6980(85)90168-6. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Pitruzzello F. J., Larsen H. S., Rouse B. T. Feedback regulation of colony-stimulating factor (CSF-1)-induced macrophage proliferation by endogenous E prostaglandins and interferon-alpha/beta. J Immunol. 1984 Aug;133(2):541–543. [PubMed] [Google Scholar]
- Newcom S. R., O'Rourke L. Potentiation of fibroblast growth by nodular sclerosing Hodgkin's disease cell cultures. Blood. 1982 Jul;60(1):228–237. [PubMed] [Google Scholar]
- Piacibello W., Rubin B. Y., Broxmeyer H. E. Prostaglandin E counteracts the gamma interferon induction of major histocompatibility complex class-II antigens on U937 cells and induction of responsiveness of U937 colony-forming cells to suppression by lactoferrin, transferrin, acidic isoferritins, and prostaglandin E. Exp Hematol. 1986 Jan;14(1):44–50. [PubMed] [Google Scholar]
- Rollins T. E., Smith W. L. Subcellular localization of prostaglandin-forming cyclooxygenase in Swiss mouse 3T3 fibroblasts by electron microscopic immunocytochemistry. J Biol Chem. 1980 May 25;255(10):4872–4875. [PubMed] [Google Scholar]
- Roth A., Kaufmann M. T., Cruchaud A., Dayer J. M. Human lymphocytes induce a differential release of prostaglandin E2 and interleukin 1-like mononuclear cell factor from normal blood monocytes. Eur J Immunol. 1985 Sep;15(9):960–963. doi: 10.1002/eji.1830150918. [DOI] [PubMed] [Google Scholar]
- Ruzicka T., Auböck J. Arachidonic acid metabolism in guinea pig Langerhans cells: studies on cyclooxygenase and lipoxygenase pathways. J Immunol. 1987 Jan 15;138(2):539–543. [PubMed] [Google Scholar]
- Salo R. J., Maddux N. L., Bleam D. K. Effect of prostaglandins on interferon synthesis in murine macrophage-like cell lines. Immunobiology. 1986 Mar;171(1-2):155–163. doi: 10.1016/S0171-2985(86)80024-9. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Rollins T. E. Characteristics of rabbit anti-PGH synthase antibodies and use in immunocytochemistry. Methods Enzymol. 1982;86:213–222. doi: 10.1016/0076-6879(82)86192-2. [DOI] [PubMed] [Google Scholar]
- Spear G. T., Marshall P., Teodorescu M. Increase in proliferation and cytotoxic cell development in human mixed lymphocyte cultures in the presence of very low concentrations of LPS: role of IL-1 and prostaglandin E2. Clin Immunol Immunopathol. 1986 Jan;38(1):32–46. doi: 10.1016/0090-1229(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Kennedy M. S., Goldyne M. E. Prostaglandin E modulation of the mitogenic response of human T cells. Differential response of T-cell subpopulations. J Clin Invest. 1979 Nov;64(5):1188–1203. doi: 10.1172/JCI109572. [DOI] [PMC free article] [PubMed] [Google Scholar]