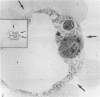
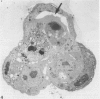
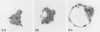Abstract
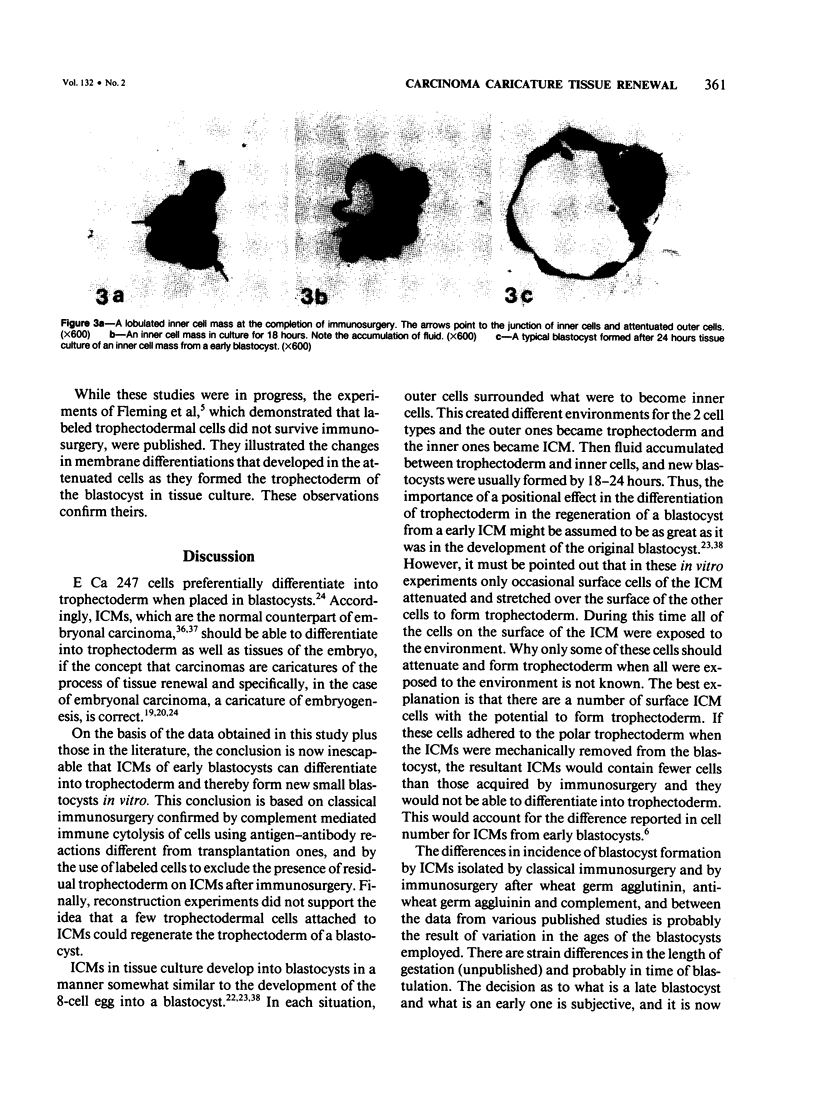
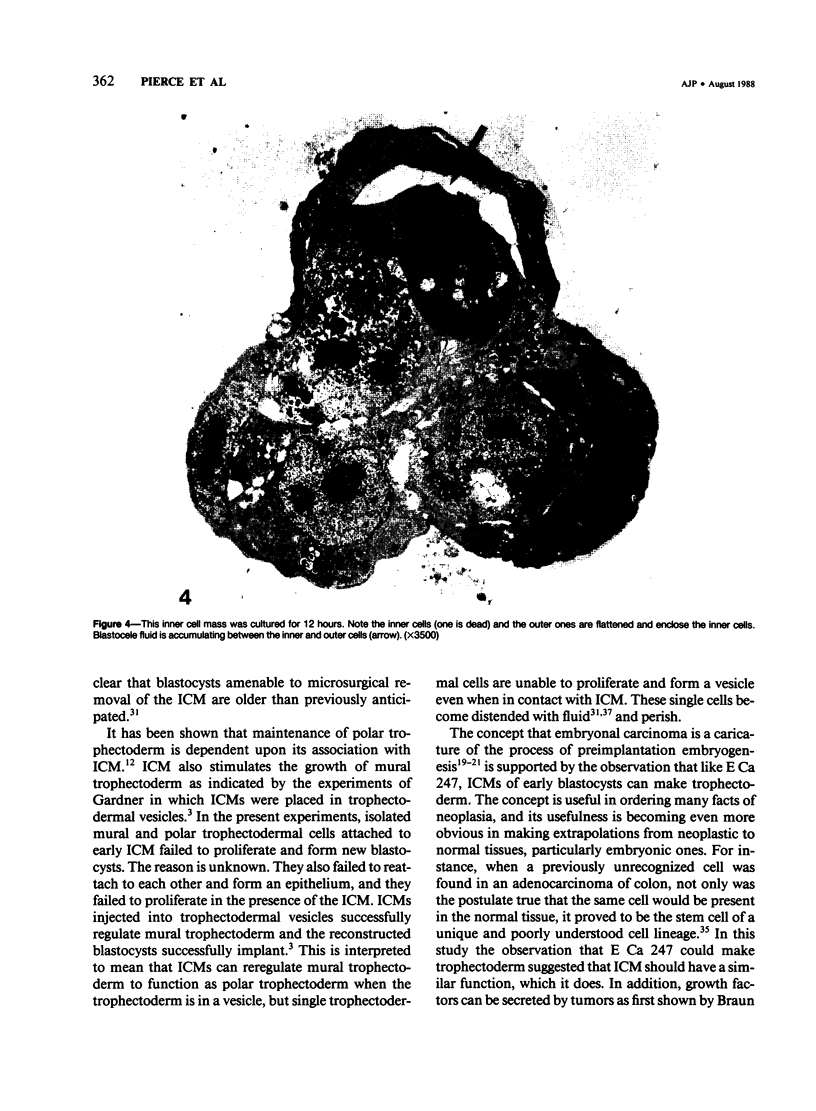
It has been established previously that when inserted in the blastocyst E Ca 247 preferentially differentiates into trophectoderm in vitro. If the concept that tumors are caricatures of the process of tissue renewal is correct, then some cells from the inner cell mass (ICM), the normal counterpart of embryonal carcinoma, should be able to differentiate into trophectoderm. This has been a controversial issue. Four experiments are now reported that support the idea that ICM can differentiate into trophectoderm: 1) ICM from early blastocysts after classical immunosurgery made blastocysts in vitro; 2) ICM obtained from early blastocysts by immunosurgery using antigens other than histocompatibility ones made blastocysts in vitro; 3) ICM from early blastocysts, in which the trophectodermal cells had been labeled, contained no labeled cells following immunosurgery; and 4) In reconstruction experiments, polar and mural trophectodermal cells attached to ICM from late blastocysts failed to multiply and make blastocysts when cultured. It is concluded that like the embryonal carcinoma some ICM cells of early blastocysts have the potential to make trophectoderm. This fact is consistent with the concept that tumors are caricatures of the process of tissue renewal; and establishes E Ca 247 as a good model for study of trophectodermal differentiation.
Full text
PDF



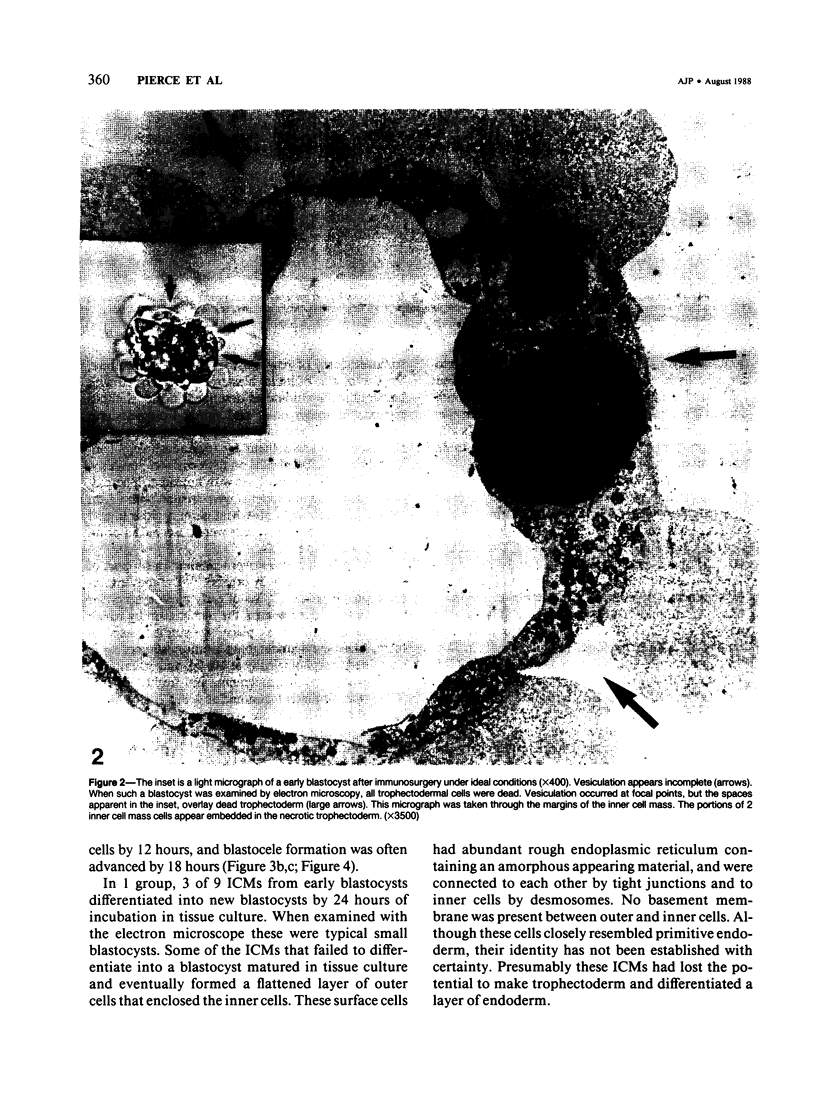


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun A. C. A DEMONSTRATION OF THE RECOVERY OF THE CROWN-GALL TUMOR CELL WITH THE USE OF COMPLEX TUMORS OF SINGLE-CELL ORIGIN. Proc Natl Acad Sci U S A. 1959 Jul;45(7):932–938. doi: 10.1073/pnas.45.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974 Oct 1;140(4):1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco P. G., Brown E. H. An ultrastructural and cytological study of preimplantation development of the mouse. J Exp Zool. 1969 Jul;171(3):253–283. doi: 10.1002/jez.1401710303. [DOI] [PubMed] [Google Scholar]
- Cox W. F., Jr, Pierce G. B. The endodermal origin of the endocrine cells of an adenocarcinoma of the colon of the rat. Cancer. 1982 Oct 15;50(8):1530–1538. doi: 10.1002/1097-0142(19821015)50:8<1530::aid-cncr2820500813>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Albertini D. F., Anderson E., Biggers J. D. The preimplantation mammalian embryo: characterization of intercellular junctions and their appearance during development. Dev Biol. 1975 Aug;45(2):231–250. doi: 10.1016/0012-1606(75)90063-9. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Warren P. D., Chisholm J. C., Johnson M. H. Trophectodermal processes regulate the expression of totipotency within the inner cell mass of the mouse expanding blastocyst. J Embryol Exp Morphol. 1984 Dec;84:63–90. [PubMed] [Google Scholar]
- Gardner R. L., Johnson M. H. An investigation of inner cell mass and trophoblast tissues following their isolation from the mouse blastocyst. J Embryol Exp Morphol. 1972 Oct;28(2):279–312. [PubMed] [Google Scholar]
- Gardner R. L., Johnson M. H. Investigation of cellular interaction and deployment in the early mammalian embryo using interspecific chimaeras between the rat and mouse. Ciba Found Symp. 1975;0(29):183–200. doi: 10.1002/9780470720110.ch9. [DOI] [PubMed] [Google Scholar]
- Gardner R. L., Johnson M. H. Investigation of early mammalian development using interspecific chimaeras between rat and mouse. Nat New Biol. 1973 Nov 21;246(151):86–89. doi: 10.1038/newbio246086a0. [DOI] [PubMed] [Google Scholar]
- Gardner R. L., Papaioannou V. E., Barton S. C. Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J Embryol Exp Morphol. 1973 Dec;30(3):561–572. [PubMed] [Google Scholar]
- Handyside A. H., Barton S. C. Evaluation of the technique of immunosurgery for the isolation of inner cell masses from mouse blastocysts. J Embryol Exp Morphol. 1977 Feb;37(1):217–226. [PubMed] [Google Scholar]
- Handyside A. H. Time of commitment of inside cells isolated from preimplantation mouse embryos. J Embryol Exp Morphol. 1978 Jun;45:37–53. [PubMed] [Google Scholar]
- Harlow G. M., Quinn P. Isolation of inner cell masses from mouse blastocysts by immunosurgery or exposure to the calcium ionophore A23187. Aust J Biol Sci. 1979 Oct;32(4-5):483–491. doi: 10.1071/bi9790483. [DOI] [PubMed] [Google Scholar]
- Hillman N., Sherman M. I., Graham C. The effect of spatial arrangement on cell determination during mouse development. J Embryol Exp Morphol. 1972 Oct;28(2):263–278. [PubMed] [Google Scholar]
- Hillman N., Tasca R. J. Ultrastructural and autoradiographic studies of mouse cleavage stages. Am J Anat. 1969 Oct;126(2):151–173. doi: 10.1002/aja.1001260203. [DOI] [PubMed] [Google Scholar]
- Hogan B., Tilly R. In vitro development of inner cell masses isolated immunosurgically from mouse blastocysts. I. Inner cell masses from 3.5-day p.c. blastocysts incubated for 24 h before immunosurgery. J Embryol Exp Morphol. 1978 Jun;45:93–105. [PubMed] [Google Scholar]
- Hogan B., Tilly R. In vitro development of inner cell masses isolated immunosurgically from mouse blastocysts. II. Inner cell masses from 3.5- to 4.0-day p.c. blastocysts. J Embryol Exp Morphol. 1978 Jun;45:107–121. [PubMed] [Google Scholar]
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadijcka M., Hillman N. Ultrastructural studies of the mouse blastocyst substages. J Embryol Exp Morphol. 1974 Dec;32(3):675–695. [PubMed] [Google Scholar]
- Nichols J., Gardner R. L. Heterogeneous differentiation of external cells in individual isolated early mouse inner cell masses in culture. J Embryol Exp Morphol. 1984 Apr;80:225–240. [PubMed] [Google Scholar]
- Papaioannou V. E., Evans E. P., Gardner R. L., Graham C. F. Growth and differentiation of an embryonal carcinoma cell line (C145b). J Embryol Exp Morphol. 1979 Dec;54:277–295. [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Arechaga J., Jones A., Lewellyn A., Wells R. S. The fate of embryonal-carcinoma cells in mouse blastocysts. Differentiation. 1987;33(3):247–253. doi: 10.1111/j.1432-0436.1987.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Lewis S. H., Miller G. J., Moritz E., Miller P. Tumorigenicity of embryonal carcinoma as an assay to study control of malignancy by the murine blastocyst. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6649–6651. doi: 10.1073/pnas.76.12.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. B. Teratocarcinoma: model for a developmental concept of cancer. Curr Top Dev Biol. 1967;2:223–246. doi: 10.1016/s0070-2153(08)60289-6. [DOI] [PubMed] [Google Scholar]
- Pierce G. B. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am J Pathol. 1983 Oct;113(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Rizzino A., Bowen-Pope D. F. Production of PDGF-like growth factors by embryonal carcinoma cells and binding of PDGF to their endoderm-like differentiated cells. Dev Biol. 1985 Jul;110(1):15–22. doi: 10.1016/0012-1606(85)90058-2. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Early mouse embryos produce and release factors with transforming growth factor activity. In Vitro Cell Dev Biol. 1985 Sep;21(9):531–536. doi: 10.1007/BF02620847. [DOI] [PubMed] [Google Scholar]
- Rossant J. Investigation of the determinative state of the mouse inner cell mass. I. Aggregation of isolated inner cell masses with morulae. J Embryol Exp Morphol. 1975 Jul;33(4):979–990. [PubMed] [Google Scholar]
- Rossant J. Investigation of the determinative state of the mouse inner cell mass. II. The fate of isolated inner cell masses transferred to the oviduct. J Embryol Exp Morphol. 1975 Jul;33(4):991–1001. [PubMed] [Google Scholar]
- Rossant J., Lis W. T. Potential of isolated mouse inner cell masses to form trophectoderm derivatives in vivo. Dev Biol. 1979 May;70(1):255–261. doi: 10.1016/0012-1606(79)90022-8. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle A. I. Trophoblast regeneration by inner cell masses isolated from cultured mouse embryos. J Exp Zool. 1978 Mar;203(3):483–489. doi: 10.1002/jez.1402030315. [DOI] [PubMed] [Google Scholar]
- Tarkowski A. K., Wróblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol. 1967 Aug;18(1):155–180. [PubMed] [Google Scholar]