Abstract
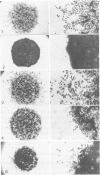
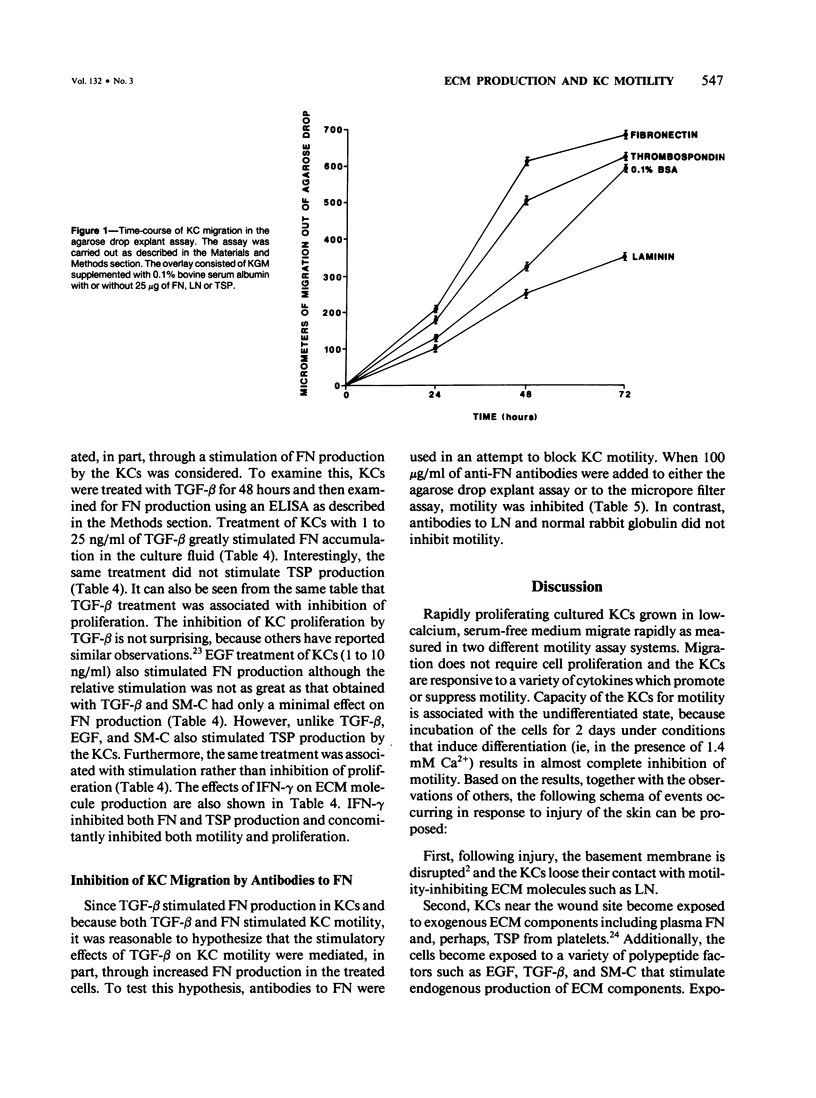
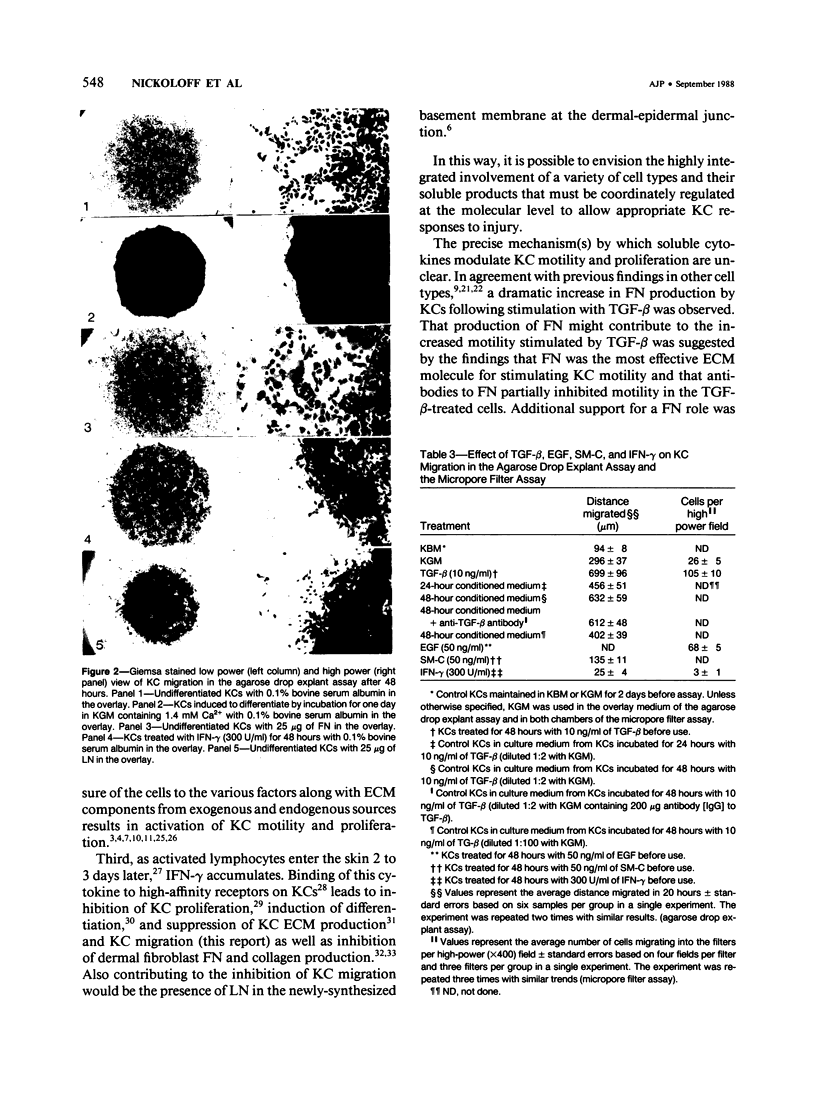
Normal human epidermal keratinocytes (KC) grown under conditions that maintain the undifferentiated state are highly motile. Migration of these cells as measured in two different assays (migration out of an agarose drop explant, and into micropore filters in a modified Boyden chamber), is stimulated by fibronectin (FN) and to a lesser extent by thrombospondin (TSP). In contrast, laminin (LN) inhibits KC migration. Cultivation of the cells for 1 day under conditions that induce differentiation (ie, in the presence of 1.4 mM Ca2+) suppresses KC motility. A number of soluble growth modulating polypeptide factors also influence KC migration. Transforming growth factor-beta (TGF-beta) and epidermal growth factor (EGF) stimulate KC motility. These factors simultaneously induce KC production of FN and a significant portion of the stimulated motility can be inhibited with antibodies to FN. EGF and somatomedin-C (SM-C), but not TGF-beta, also stimulate TSP production while EGF and SM-C (but not TGF-beta) induce KC proliferation. In contrast to these factors, interferon-gamma (INF-gamma) inhibits KC production of both FN and TSP and concomitantly inhibits both motility and proliferation. These data suggest that KC properties essential for normal wound healing (ie, motility and proliferation) are regulated by both extracellular matrix molecules and soluble peptide factors. Finally, these effects of various growth promoting and antiproliferative factors on KCs may, in part, be mediated through alteration in the endogenous production of extracellular matrix molecules by KCs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965 Dec;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Galvin N. J., O'Rourke K. M., Frazier W. A. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986 Feb 5;261(4):1962–1968. [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Fyrand O. Studies on fibronectin in the skin. II. Indirect immunofluorescence studies in psoriasis vulgaris. Arch Dermatol Res. 1979 Aug;266(1):33–41. doi: 10.1007/BF00412860. [DOI] [PubMed] [Google Scholar]
- Granstein R. D., Murphy G. F., Margolis R. J., Byrne M. H., Amento E. P. Gamma-interferon inhibits collagen synthesis in vivo in the mouse. J Clin Invest. 1987 Apr;79(4):1254–1258. doi: 10.1172/JCI112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jimenez S. A., Freundlich B., Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984 Sep;74(3):1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Kan M., Isemura M., Yamane I., Tagami H. Effects of extracellular matrices on human keratinocyte adhesion and growth and on its secretion and deposition of fibronectin in culture. J Invest Dermatol. 1987 May;88(5):594–601. doi: 10.1111/1523-1747.ep12470207. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Karasek M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J Invest Dermatol. 1978 Aug;71(2):157–162. doi: 10.1111/1523-1747.ep12546943. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R., Nishikawa T. Active epidermal movement in human skin in vitro. Br J Dermatol. 1973 Mar;88(3):245–248. doi: 10.1111/j.1365-2133.1973.tb07542.x. [DOI] [PubMed] [Google Scholar]
- Mommaas-Kienhuis A. M., Grayson S., Wijsman M. C., Vermeer B. J., Elias P. M. Low density lipoprotein receptor expression on keratinocytes in normal and psoriatic epidermis. J Invest Dermatol. 1987 Nov;89(5):513–517. doi: 10.1111/1523-1747.ep12461024. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., Magid M., King L. E., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986 Mar;86(3):260–265. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Basham T. Y., Merigan T. C., Morhenn V. B. Antiproliferative effects of recombinant alpha- and gamma-interferons on cultured human keratinocytes. Lab Invest. 1984 Dec;51(6):697–701. [PubMed] [Google Scholar]
- Nickoloff B. J., Mahrle G., Morhenn V. Ultrastructural effects of recombinant gamma-interferon on cultured human keratinocytes. Ultrastruct Pathol. 1986;10(1):17–21. doi: 10.3109/01913128609015559. [DOI] [PubMed] [Google Scholar]
- O'Keefe E. J., Payne R. E., Jr, Russell N., Woodley D. T. Spreading and enhanced motility of human keratinocytes on fibronectin. J Invest Dermatol. 1985 Aug;85(2):125–130. doi: 10.1111/1523-1747.ep12276531. [DOI] [PubMed] [Google Scholar]
- Odland G., Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968 Oct;39(1):135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Pittelkow M. R., Wille J. J., Jr, Scott R. E., Moses H. L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Toda K., Tuan T. L., Brown P. J., Grinnell F. Fibronectin receptors of human keratinocytes and their expression during cell culture. J Cell Biol. 1987 Dec;105(6 Pt 2):3097–3104. doi: 10.1083/jcb.105.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. A comparison of the migration patterns of normal and malignant cells in two assay systems. Am J Pathol. 1978 Jan;90(1):159–172. [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., O'Keefe E. J., Prunieras M. Cutaneous wound healing: a model for cell-matrix interactions. J Am Acad Dermatol. 1985 Feb;12(2 Pt 2):420–433. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]