Abstract
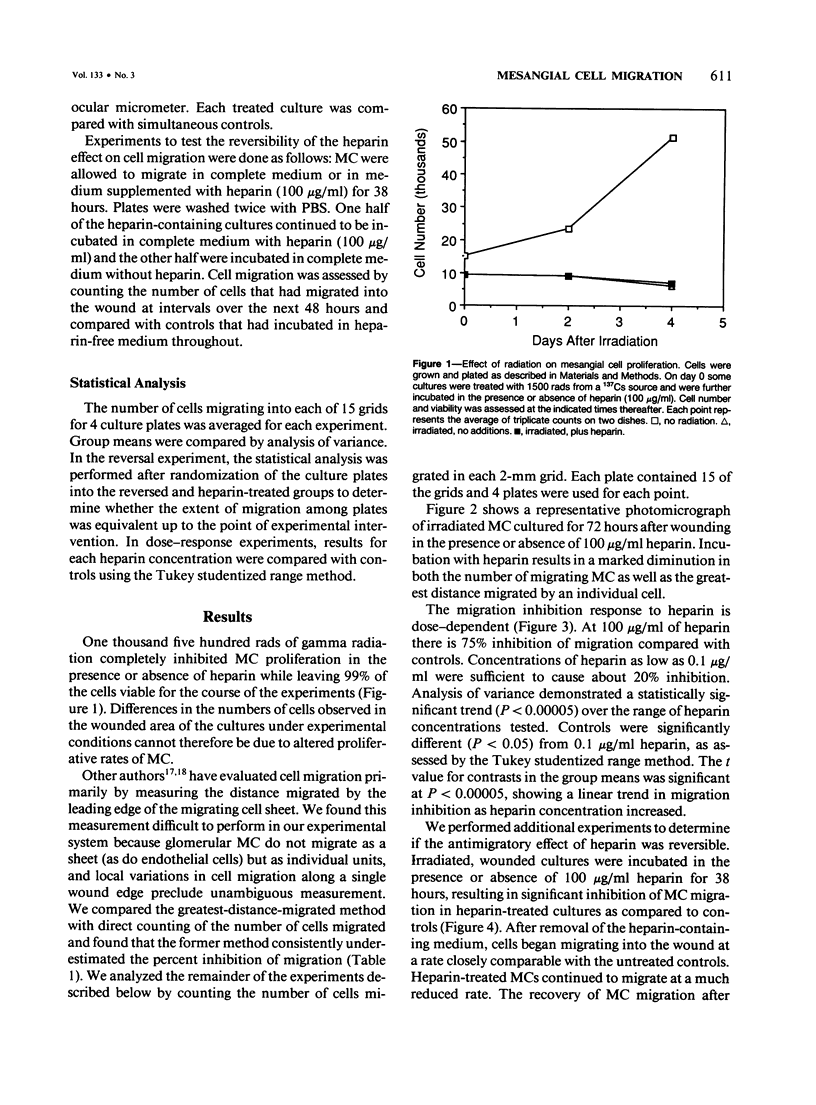
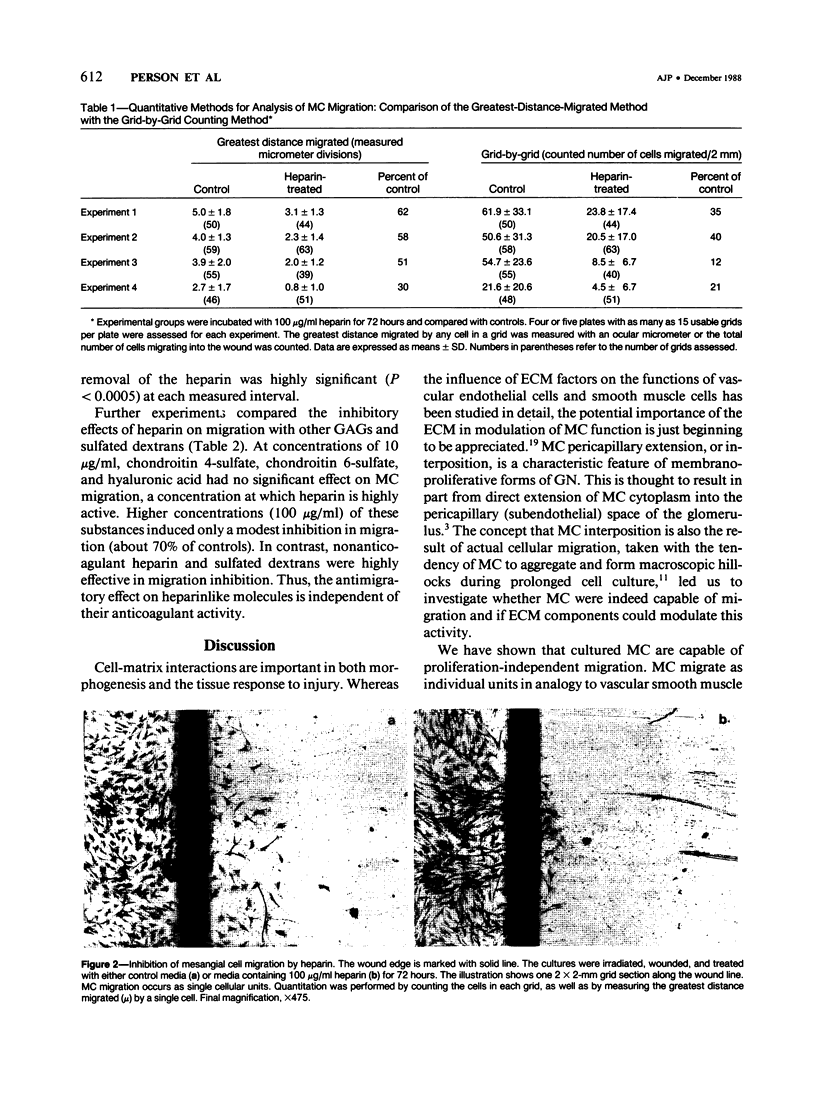
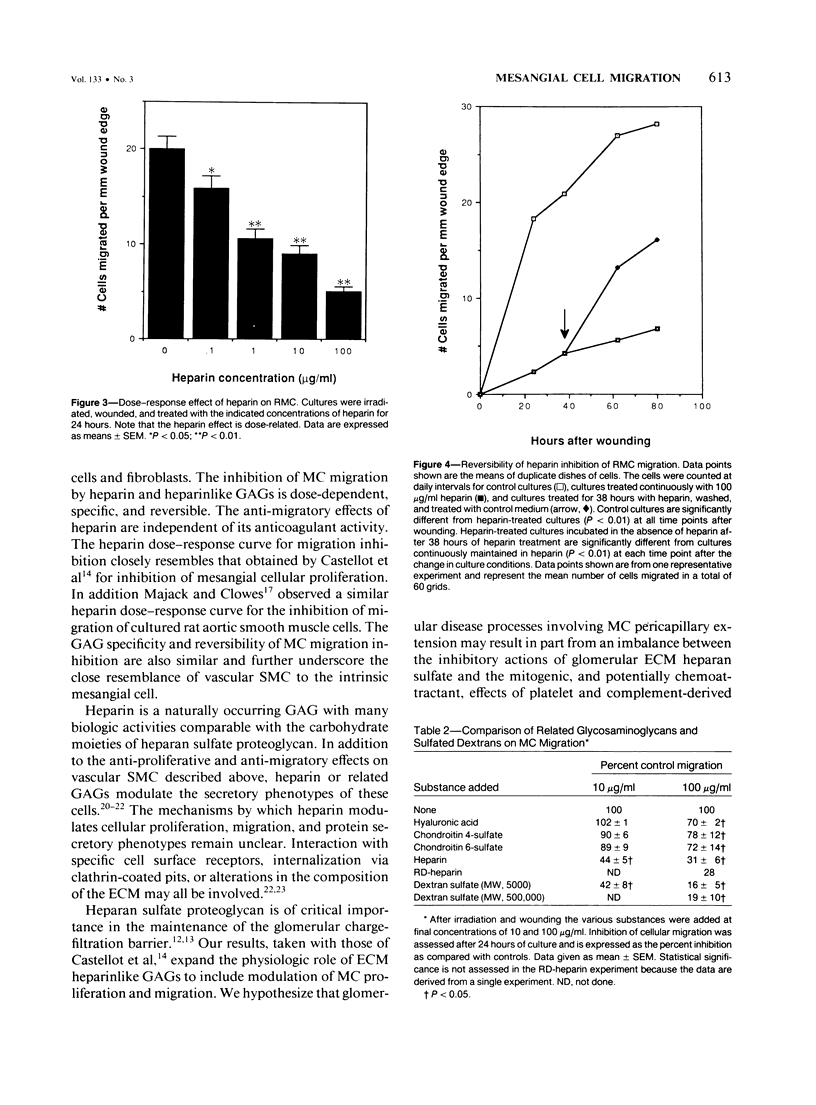
Extension of mesangial cells (MC) into the pericapillary space is a pathologic response seen in several forms of glomerulonephritis. This process may involve both cytoplasmic extension by MC and actual cellular migration. For investigation of whether extracellular matrix factors could modulate this process, the migratory responses of rat MC were quantitatively examined using a cell culture model. Denuding ("wounding") a portion of a confluent culture of MC was followed by migration of mesangial cells into the denuded area. The expected proliferative response to this treatment was blocked by irradiation. The migratory response began within 8 hours of wounding and continued for at least 80 hours. The MC migratory response was specifically inhibited in a dose-dependent and reversible manner by heparin and heparinlike glycosaminoglycans (GAGs). Chondroitin sulfates and hyaluronic acid did not significantly inhibit MC migration. Glomerular basement membrane heparinlike GAGs may normally prevent MC extension into the pericapillary space. Changes in the density or composition of these substances during glomerular inflammatory processes could permit the development of MC pericapillary extensions and thereby lead to further alterations in basement membrane integrity.
Full text
PDF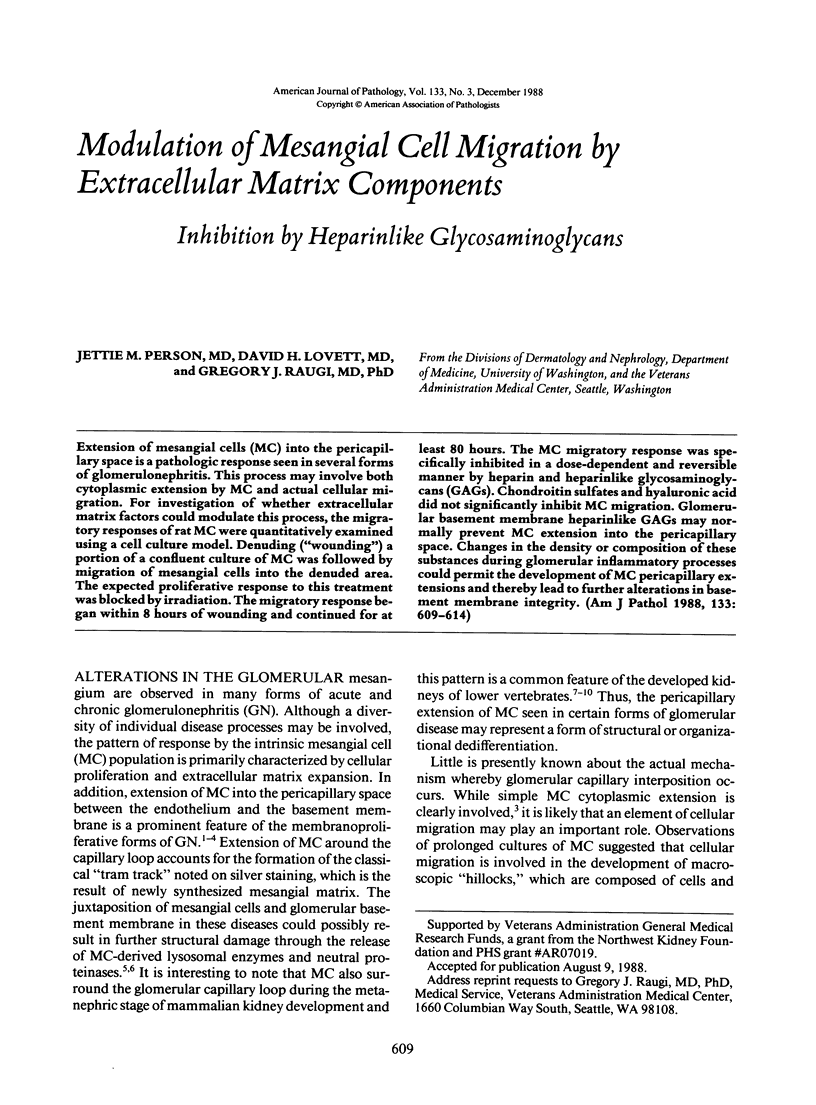


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellot J. J., Jr, Favreau L. V., Karnovsky M. J., Rosenberg R. D. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982 Oct 10;257(19):11256–11260. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Hoover R. L., Harper P. A., Karnovsky M. J. Heparin and glomerular epithelial cell-secreted heparin-like species inhibit mesangial-cell proliferation. Am J Pathol. 1985 Sep;120(3):427–435. [PMC free article] [PubMed] [Google Scholar]
- Churg J., Grishman E. Ultrastructure of glomerular disease: a review. Kidney Int. 1975 Apr;7(4):254–261. doi: 10.1038/ki.1975.37. [DOI] [PubMed] [Google Scholar]
- Coffey A. K., Karnovsky M. J. Heparin inhibits mesangial cell proliferation in habu-venom-induced glomerular injury. Am J Pathol. 1985 Aug;120(2):248–255. [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Cavallo T. Membranoproliferative glomerulonephritis. Localization of early components of complement in glomerular deposits. Am J Pathol. 1976 Aug;84(2):283–298. [PMC free article] [PubMed] [Google Scholar]
- Habib R., Kleinknecht C., Gubler M. C., Levy M. Idiopathic membranoproliferative glomerulonephritis in children. Report of 105 cases. Clin Nephrol. 1973 Jul-Aug;1(4):194–214. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L., Rosenzweig L. J. Distribution of sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Eur J Cell Biol. 1983 Sep;31(2):290–295. [PubMed] [Google Scholar]
- Kashgarian M. Mesangium and glomerular disease. Lab Invest. 1985 Jun;52(6):569–571. [PubMed] [Google Scholar]
- Kazimierczak J. A study of scanning (SEM) and transmission (TEM) electron microscopy of the glomerular capillaries in developing rat kidney. Cell Tissue Res. 1980;212(2):241–255. doi: 10.1007/BF00233959. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Kashgarian M., Sterzel R. B. Lysosomal enzymes in glomerular cells of the rat. Am J Pathol. 1982 May;107(2):161–166. [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Kashgarian M., Ryan J. L. Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int. 1983 Feb;23(2):342–349. doi: 10.1038/ki.1983.25. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Bornstein P. Heparin and related glycosaminoglycans modulate the secretory phenotype of vascular smooth muscle cells. J Cell Biol. 1984 Nov;99(5):1688–1695. doi: 10.1083/jcb.99.5.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Bornstein P. Heparin regulates the collagen phenotype of vascular smooth muscle cells: induced synthesis of an Mr 60,000 collagen. J Cell Biol. 1985 Feb;100(2):613–619. doi: 10.1083/jcb.100.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osathanondh V., Potter E. L. Development of human kidney as shown by microdissection. V. Development of vascular pattern of glomerulus. Arch Pathol. 1966 Nov;82(5):403–411. [PubMed] [Google Scholar]
- PAK POY R. K. Electron microscopy of the amphibian renal glomerulus. Aust J Exp Biol Med Sci. 1957 Dec;35(6):583–593. doi: 10.1038/icb.1957.60. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Lovett D. H. Thrombospondin secretion by cultured human glomerular mesangial cells. Am J Pathol. 1987 Nov;129(2):364–372. [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Foellmer H. G., Perfetto M., Biemesderfer D., Kashgarian M. Mesangial cell hillocks. Nodular foci of exaggerated growth of cells and matrix in prolonged culture. Am J Pathol. 1986 Oct;125(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Stein H. D., Kashgarian M. The mesangium and glomerulonephritis. Klin Wochenschr. 1982 Sep 15;60(18):1077–1094. doi: 10.1007/BF01715838. [DOI] [PubMed] [Google Scholar]



