Abstract
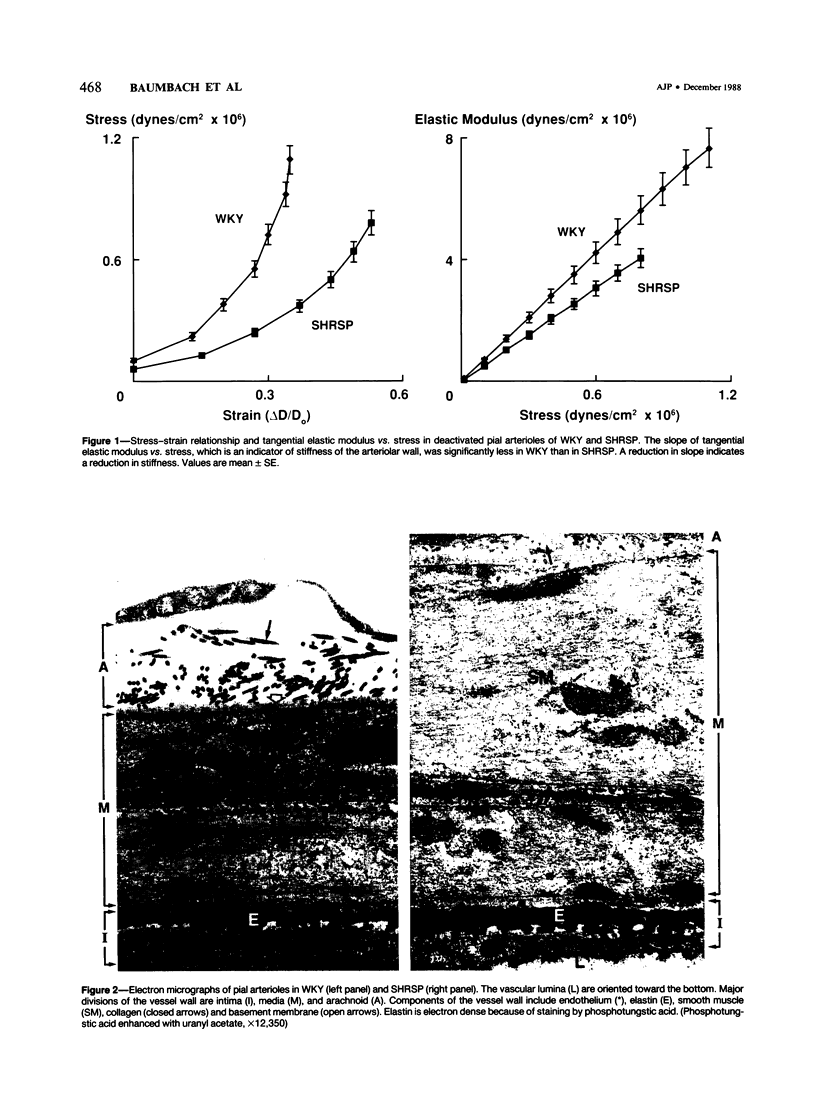
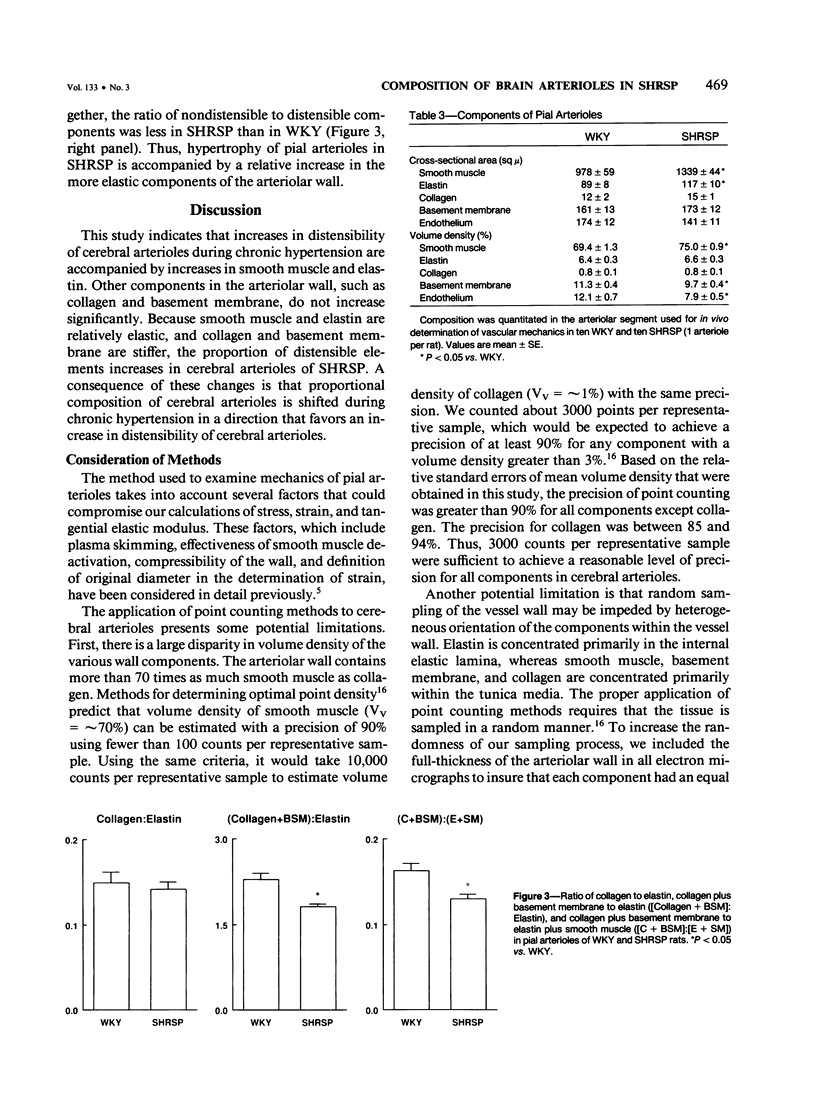
It was demonstrated recently that, in contrast to large cerebral arteries, distensibility of cerebral arterioles is increased in stroke-prone spontaneously hypertensive rats (SHRSP). The goals of this study were to examine composition of normal cerebral arterioles, and to determine whether chronic hypertension alters relative composition of the arteriolar wall. Pial arterioles in normotensive Wistar Kyoto rats contain large amounts of smooth muscle, small amounts of elastin and basement membrane, and very little collagen. Hypertrophy of pial arterioles in SHRSP is characterized by increases in the elastic components, smooth muscle and elastin. The stiffer components, collagen and basement membrane either did not change or decreased. It is concluded that cerebral arterioles contain proportionately more smooth muscle and less collagen than large arteries, and that hypertrophy of cerebral arterioles in SHRSP is accompanied by a relative increase in the more elastic components of the arteriolar wall, which probably contributes to the increase in arteriolar distensibility.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R. Recent studies on the structure and pathology of basement membranes. J Pathol. 1986 Aug;149(4):257–278. doi: 10.1002/path.1711490402. [DOI] [PubMed] [Google Scholar]
- Baumbach G. L., Dobrin P. B., Hart M. N., Heistad D. D. Mechanics of cerebral arterioles in hypertensive rats. Circ Res. 1988 Jan;62(1):56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- Bergel D. H. The static elastic properties of the arterial wall. J Physiol. 1961 May;156(3):445–457. doi: 10.1113/jphysiol.1961.sp006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Halpern W., Brann L. R. Biochemical and mechanical properties of resistance arteries from normotensive and hypertensive rats. Hypertension. 1983 Jan-Feb;5(1):17–25. doi: 10.1161/01.hyp.5.1.17. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Vaishnav R. N., Patel D. J. Compressibility of the arterial wall. Circ Res. 1968 Jul;23(1):61–68. doi: 10.1161/01.res.23.1.61. [DOI] [PubMed] [Google Scholar]
- Cox R. H. Mechanics of canine iliac artery smooth muscle in vitro. Am J Physiol. 1976 Feb;230(2):462–470. doi: 10.1152/ajplegacy.1976.230.2.462. [DOI] [PubMed] [Google Scholar]
- Dobrin P. B., Rovick A. A. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969 Dec;217(6):1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Llaurado J. G. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966 Aug;19(2):394–399. doi: 10.1161/01.res.19.2.394. [DOI] [PubMed] [Google Scholar]
- Fung Y. C. Elasticity of soft tissues in simple elongation. Am J Physiol. 1967 Dec;213(6):1532–1544. doi: 10.1152/ajplegacy.1967.213.6.1532. [DOI] [PubMed] [Google Scholar]
- Harper S. L., Bohlen H. G. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984 May-Jun;6(3):408–419. doi: 10.1161/01.hyp.6.3.408. [DOI] [PubMed] [Google Scholar]
- Kühn K., Glanville R. W., Babel W., Qian R. Q., Dieringer H., Voss T., Siebold B., Oberbäumer I., Schwarz U., Yamada Y. The structure of type IV collagen. Ann N Y Acad Sci. 1985;460:14–24. doi: 10.1111/j.1749-6632.1985.tb51153.x. [DOI] [PubMed] [Google Scholar]
- Mayne R. Collagenous proteins of blood vessels. Arteriosclerosis. 1986 Nov-Dec;6(6):585–593. doi: 10.1161/01.atv.6.6.585. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Bohlen H. G., Miller B. G., Evan A. P. Cellular and vessel wall morphology of cerebral cortical arterioles after short-term diabetes in adult rats. Blood Vessels. 1985;22(6):265–277. doi: 10.1159/000158613. [DOI] [PubMed] [Google Scholar]
- Stromberg D. D., Wiederhielm C. A. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969 Jun;26(6):857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- Timpl R., Oberbäumer I., von der Mark H., Bode W., Wick G., Weber S., Engel J. Structure and biology of the globular domain of basement membrane type IV collagen. Ann N Y Acad Sci. 1985;460:58–72. doi: 10.1111/j.1749-6632.1985.tb51157.x. [DOI] [PubMed] [Google Scholar]
- Toda N., Okunishi H., Miyazaki M. Length-passive tension relationships in cerebral and peripheral arteries isolated from spontaneously hypertensive and normotensive rats. Jpn Circ J. 1982 Oct;46(10):1088–1094. doi: 10.1253/jcj.46.1088. [DOI] [PubMed] [Google Scholar]
- Walmsley J. G., Gore R. W., Dacey R. G., Jr, Damon D. N., Duling B. R. Quantitative morphology of arterioles from the hamster cheek pouch related to mechanical analysis. Microvasc Res. 1982 Nov;24(3):249–271. doi: 10.1016/0026-2862(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Wiederhielm C. A. Distensibility characteristics of small blood vessels. Fed Proc. 1965 Sep-Oct;24(5):1075–1084. [PubMed] [Google Scholar]
- Winquist R. J., Bohr D. F. Structural and functional changes in cerebral arteries from spontaneously hypertensive rats. Hypertension. 1983 May-Jun;5(3):292–297. doi: 10.1161/01.hyp.5.3.292. [DOI] [PubMed] [Google Scholar]



