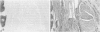Abstract
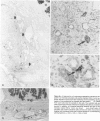
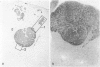
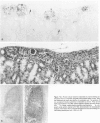
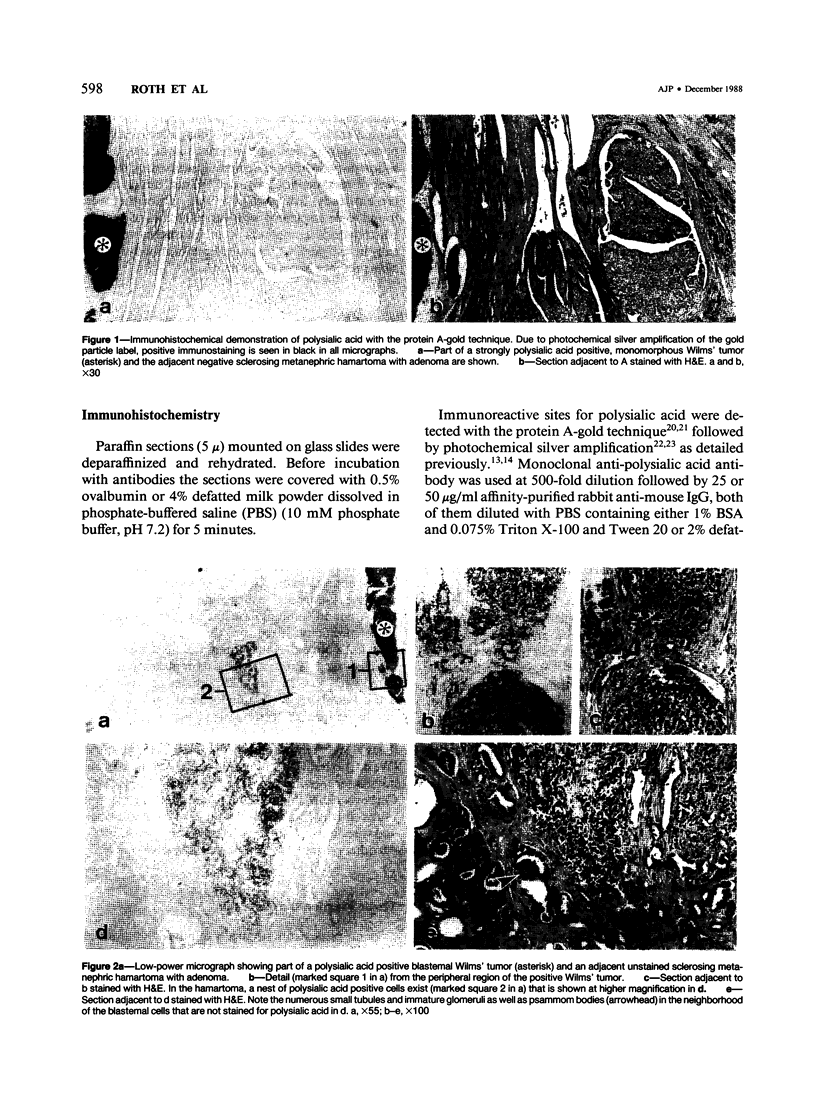
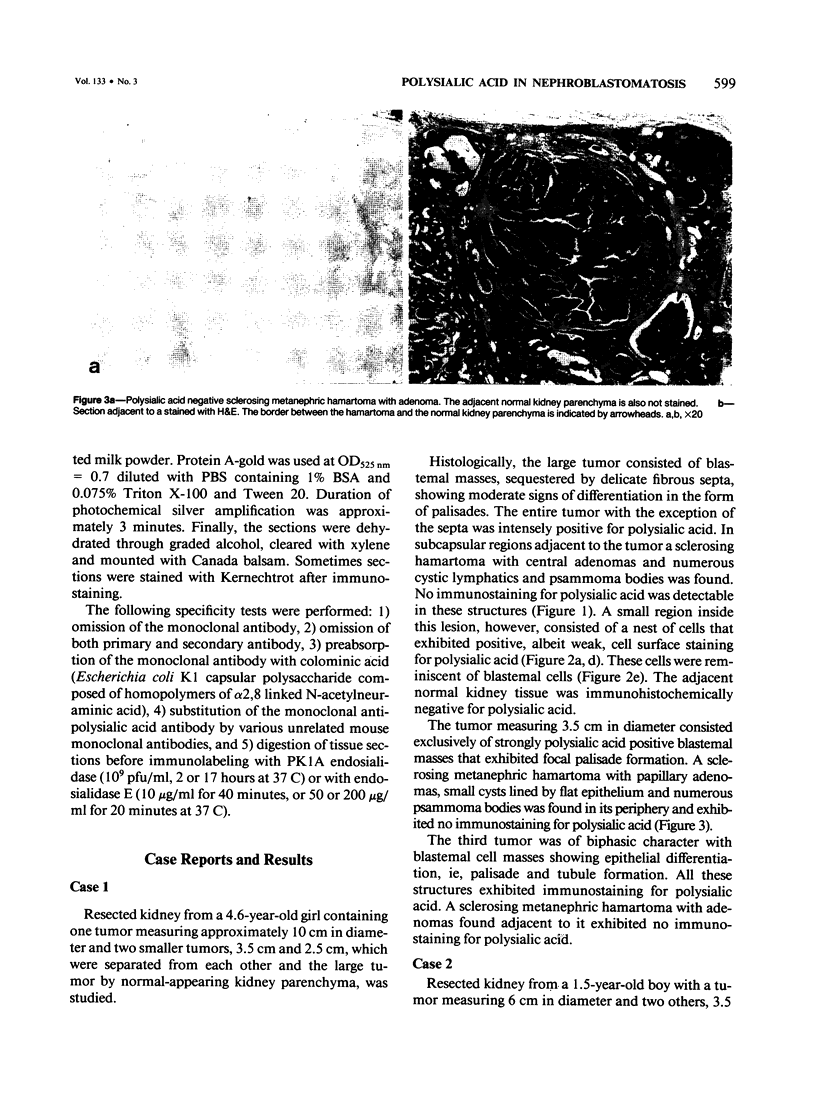
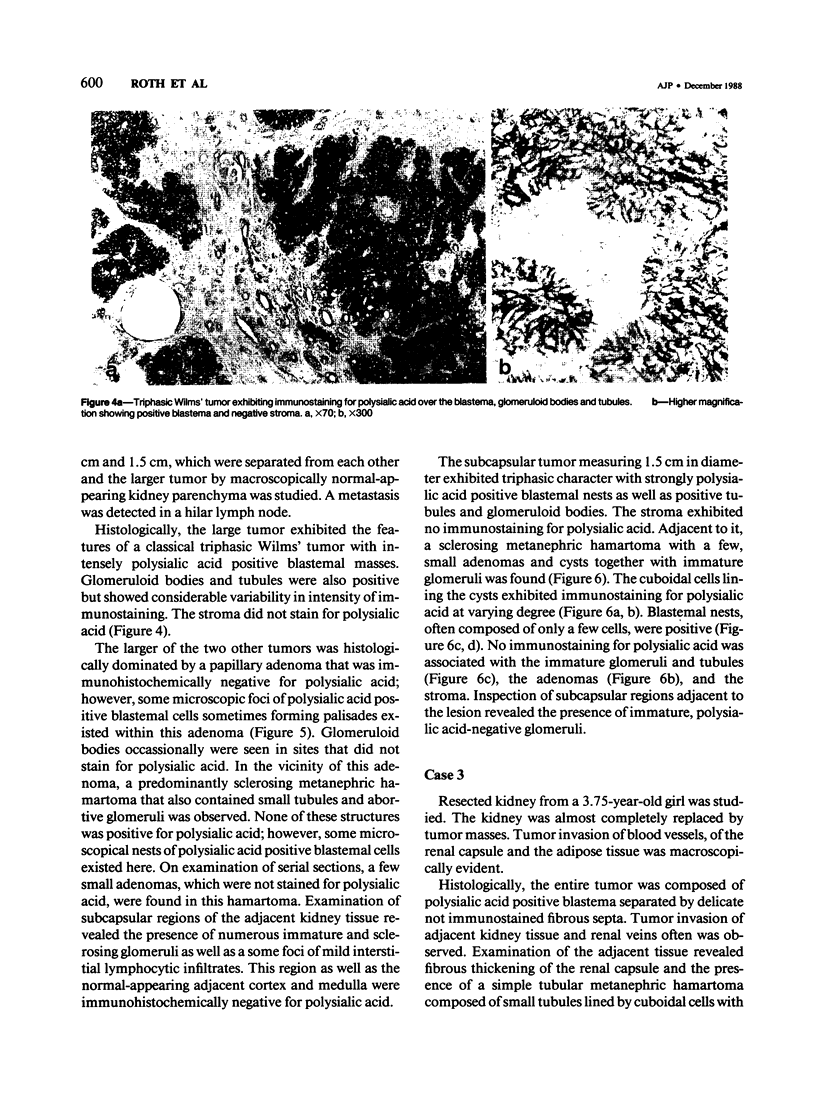
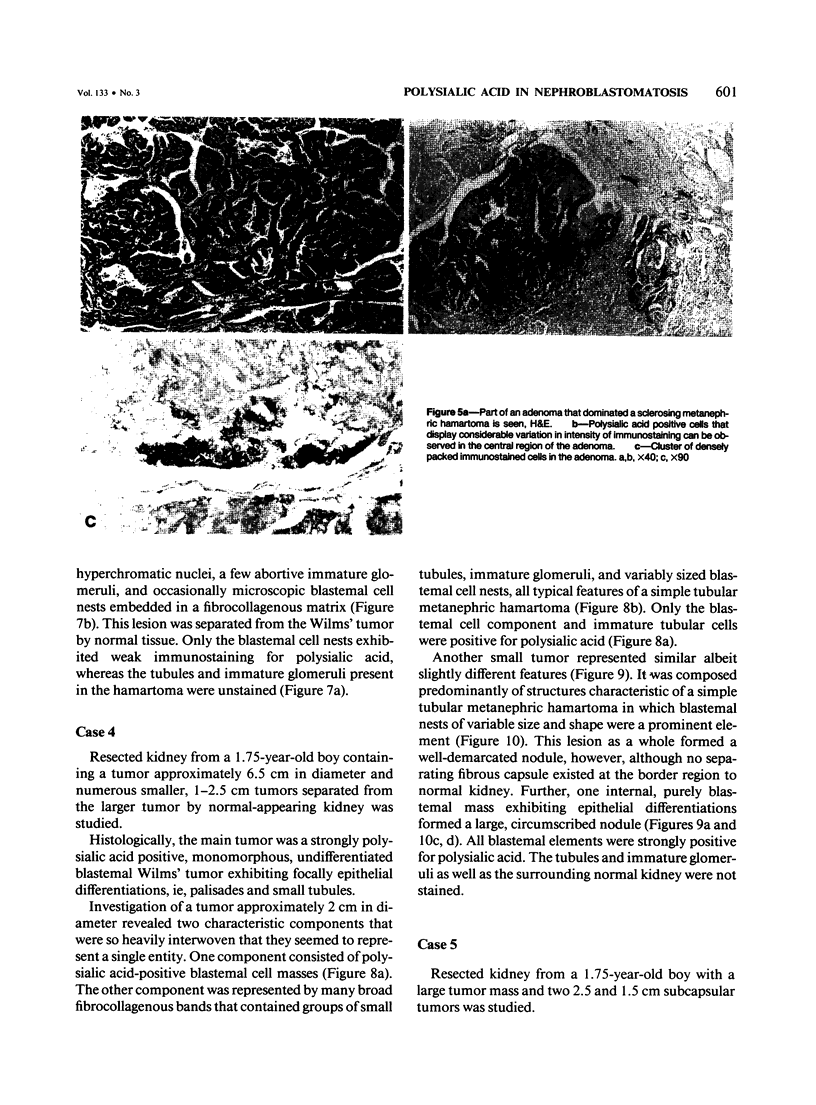
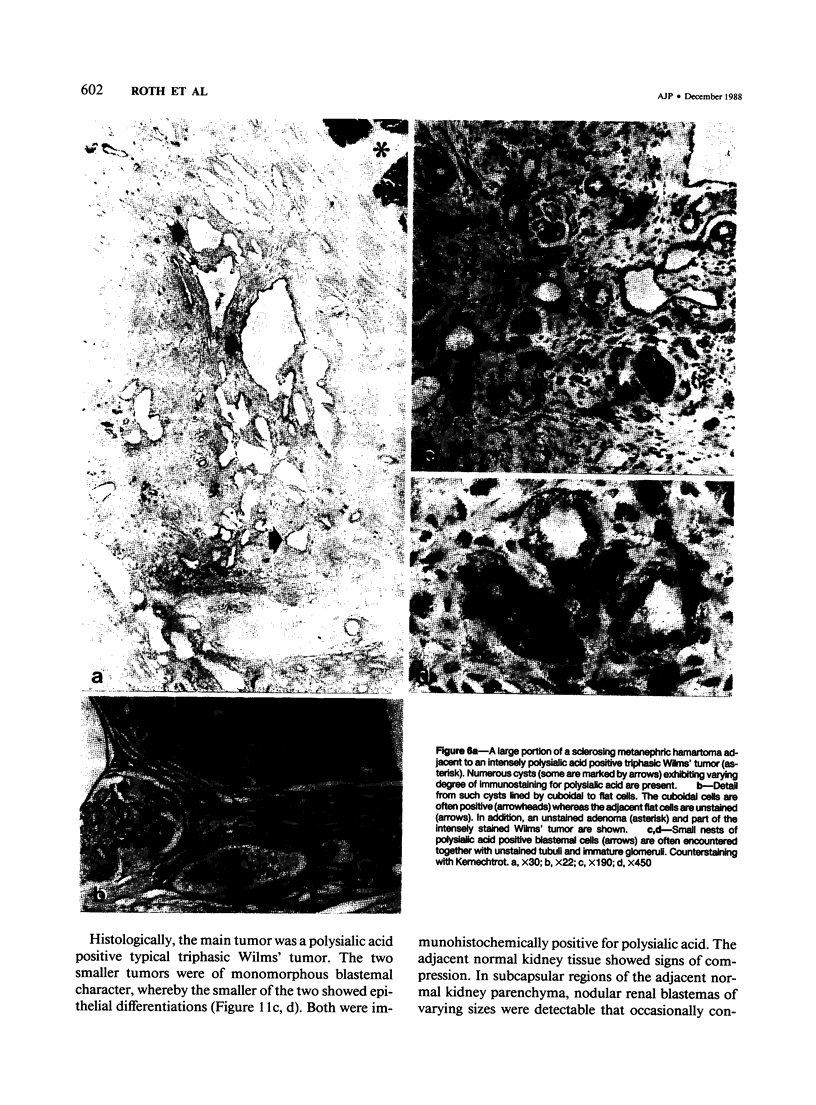
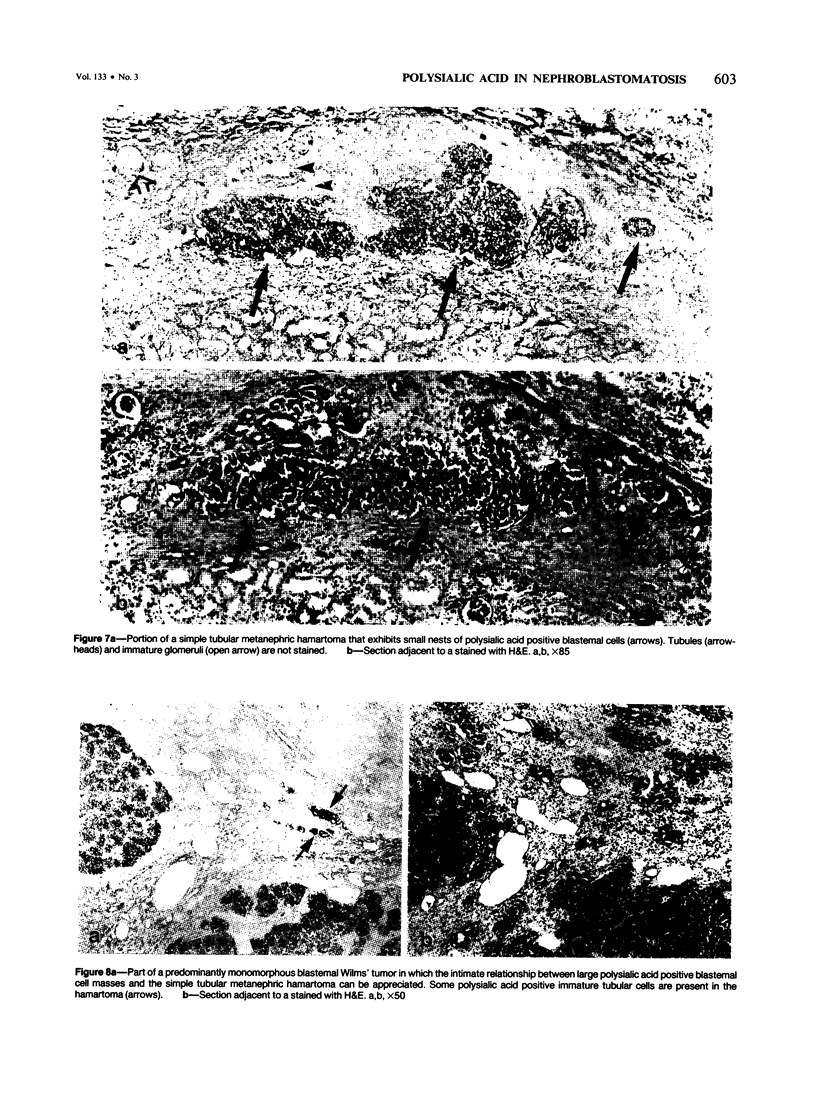
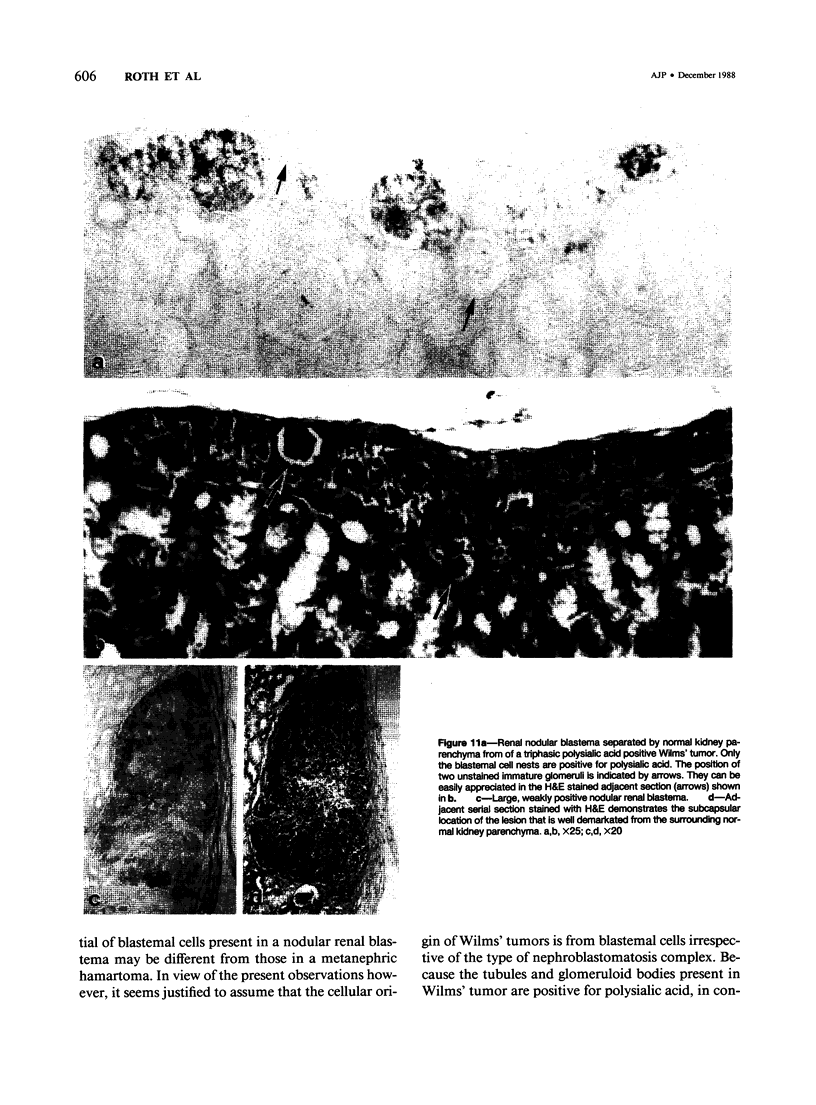
Previous investigations on polysialic acid of the neural cell adhesion molecule NCAM in human kidney have demonstrated its presence during nephrogenesis in embryonic kidney, absence in normal adult kidney, and reexpression in Wilms' tumor. These data showed that polysialic acid of NCAM is an onco-developmental antigen in human kidney and provided more direct evidence for the metanephric origin of Wilms' tumor. In the present study, five cases of Wilms' tumor associated with nephroblastomatosis complexes were immunohistochemically investigated with a monoclonal antibody for the presence of polysialic acid. Regardless of the type of nephroblastomatosis complex, ie, renal nodular blastema, simple tubular metanephric hamartoma, sclerosing metanephric hamartoma with adenoma, or incipient Wilms' tumor, immunoreactivity for polysialic acid was found in the blastemal cells, but was undetectable in all other structural elements. Because only blastemal cells exhibited a characteristic feature of embryonal differentiating metanephric derivatives, it appears that Wilms' tumor has its origin not exclusively in nodular renal blastema but rather in blastemal cells present in the various forms of nephroblastomatosis complex. The presence of polysialic acid of NCAM in blastemal cells in such lesions indicates that further events in addition to the expression of the embryonic form of this cell adhesion molecule may be involved in the pathogenesis of Wilms' tumor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., DePinho R., Zimmerman K., Legouy E., Hatton K., Ferrier P., Tesfaye A., Yancopoulos G., Nisen P. The human myc gene family. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):931–941. doi: 10.1101/sqb.1986.051.01.106. [DOI] [PubMed] [Google Scholar]
- Ashley D. J. The two "hit" and multiple "hit" theories of carcinogenesis. Br J Cancer. 1969 Jun;23(2):313–328. doi: 10.1038/bjc.1969.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove K. E., McAdams A. J. The nephroblastomatosis complex and its relationship to Wilms' tumor: a clinicopathologic treatise. Perspect Pediatr Pathol. 1976;3:185–223. [PubMed] [Google Scholar]
- Finne J., Bitter-Suermann D., Goridis C., Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987 Jun 15;138(12):4402–4407. [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Francke U., Holmes L. B., Atkins L., Riccardi V. M. Aniridia-Wilms' tumor association: evidence for specific deletion of 11p13. Cytogenet Cell Genet. 1979;24(3):185–192. doi: 10.1159/000131375. [DOI] [PubMed] [Google Scholar]
- Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman R. L., Haase G. M., Foley C. L., Wilson H. L., Bailey W. C. Nephroblastomatosis and Wilms' tumor. Clinical experience and management of seven patients. Cancer. 1985 Apr 1;55(7):1446–1451. doi: 10.1002/1097-0142(19850401)55:7<1446::aid-cncr2820550704>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Machin G. A. Persistent renal blastema (nephroblastomatosis) as a frequent precursor of Wilms' tumor; a pathological and clinical review. Part 2. Significance of nephroblastomatosis in the genesis of Wilms' tumor. Am J Pediatr Hematol Oncol. 1980 Fall;2(3):253–261. [PubMed] [Google Scholar]
- Manigley C., Roth J. Applications of immunocolloids in light microscopy. IV. Use of photochemical silver staining in a simple and efficient double-staining technique. J Histochem Cytochem. 1985 Dec;33(12):1247–1251. doi: 10.1177/33.12.2415576. [DOI] [PubMed] [Google Scholar]
- Mierau G. W., Beckwith J. B., Weeks D. A. Ultrastructure and histogenesis of the renal tumors of childhood: an overview. Ultrastruct Pathol. 1987;11(2-3):313–333. doi: 10.3109/01913128709048329. [DOI] [PubMed] [Google Scholar]
- Morton C. C., Byers M. G., Nakai H., Bell G. I., Shows T. B. Human genes for insulin-like growth factors I and II and epidermal growth factor are located on 12q22----q24.1, 11p15, and 4q25----q27, respectively. Cytogenet Cell Genet. 1986;41(4):245–249. doi: 10.1159/000132237. [DOI] [PubMed] [Google Scholar]
- Nguyen C., Mattei M. G., Mattei J. F., Santoni M. J., Goridis C., Jordan B. R. Localization of the human NCAM gene to band q23 of chromosome 11: the third gene coding for a cell interaction molecule mapped to the distal portion of the long arm of chromosome 11. J Cell Biol. 1986 Mar;102(3):711–715. doi: 10.1083/jcb.102.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisen P. D., Zimmerman K. A., Cotter S. V., Gilbert F., Alt F. W. Enhanced expression of the N-myc gene in Wilms' tumors. Cancer Res. 1986 Dec;46(12 Pt 1):6217–6222. [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Riccardi V. M., Sujansky E., Smith A. C., Francke U. Chromosomal imbalance in the Aniridia-Wilms' tumor association: 11p interstitial deletion. Pediatrics. 1978 Apr;61(4):604–610. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Bitter-Suermann D., Finne J. Polysialic acid units are spatially and temporally expressed in developing postnatal rat kidney. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1969–1973. doi: 10.1073/pnas.84.7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Zuber C., Wagner P., Blaha I., Bitter-Suermann D., Heitz P. U. Presence of the long chain form of polysialic acid of the neural cell adhesion molecule in Wilms' tumor. Identification of a cell adhesion molecule as an oncodevelopmental antigen and implications for tumor histogenesis. Am J Pathol. 1988 Nov;133(2):227–240. [PMC free article] [PubMed] [Google Scholar]
- Roth J., Zuber C., Wagner P., Taatjes D. J., Weisgerber C., Heitz P. U., Goridis C., Bitter-Suermann D. Reexpression of poly(sialic acid) units of the neural cell adhesion molecule in Wilms tumor. Proc Natl Acad Sci U S A. 1988 May;85(9):2999–3003. doi: 10.1073/pnas.85.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Dickersin G. R., Vawter G. F., Mackay B., Harms D. Wilms' tumor: review of ultrastructure and histogenesis. Pathobiol Annu. 1982;12:281–300. [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Davis L. M., Qin S., Nowak N. J. The chromosome 11 gene map: genes for growth and development, Wilms' tumor deletions, and cancer chromosome breakpoints. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):867–877. doi: 10.1101/sqb.1986.051.01.100. [DOI] [PubMed] [Google Scholar]
- Taatjes D. J., Schaub U., Roth J. Light microscopical detection of antigens and lectin binding sites with gold-labelled reagents on semi-thin Lowicryl K4M sections: usefulness of the photochemical silver reaction for signal amplification. Histochem J. 1987 Apr;19(4):235–245. doi: 10.1007/BF01680634. [DOI] [PubMed] [Google Scholar]
- Tomlinson S., Taylor P. W. Neuraminidase associated with coliphage E that specifically depolymerizes the Escherichia coli K1 capsular polysaccharide. J Virol. 1985 Aug;55(2):374–378. doi: 10.1128/jvi.55.2.374-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman K. A., Yancopoulos G. D., Collum R. G., Smith R. K., Kohl N. E., Denis K. A., Nau M. M., Witte O. N., Toran-Allerand D., Gee C. E. Differential expression of myc family genes during murine development. 1986 Feb 27-Mar 5Nature. 319(6056):780–783. doi: 10.1038/319780a0. [DOI] [PubMed] [Google Scholar]
- de Chadarévian J. P., Fletcher B. D., Chatten J., Rabinovitch H. H. Massive infantile nephroblastomatosis: a clinical, radiological, and pathological analysis of four cases. Cancer. 1977 May;39(5):2294–2305. doi: 10.1002/1097-0142(197705)39:5<2294::aid-cncr2820390551>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]