Abstract
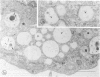
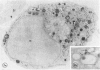
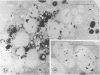
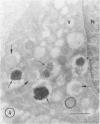
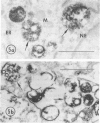
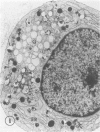
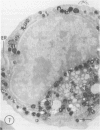
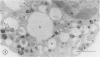
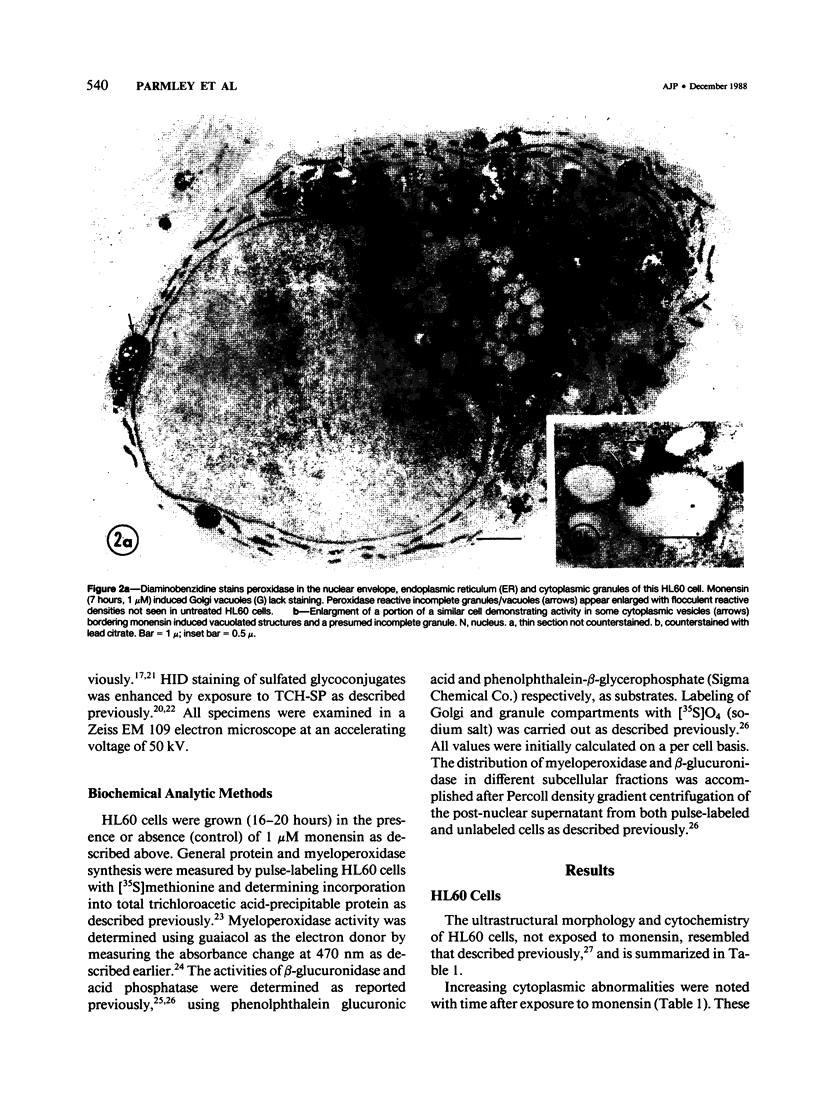
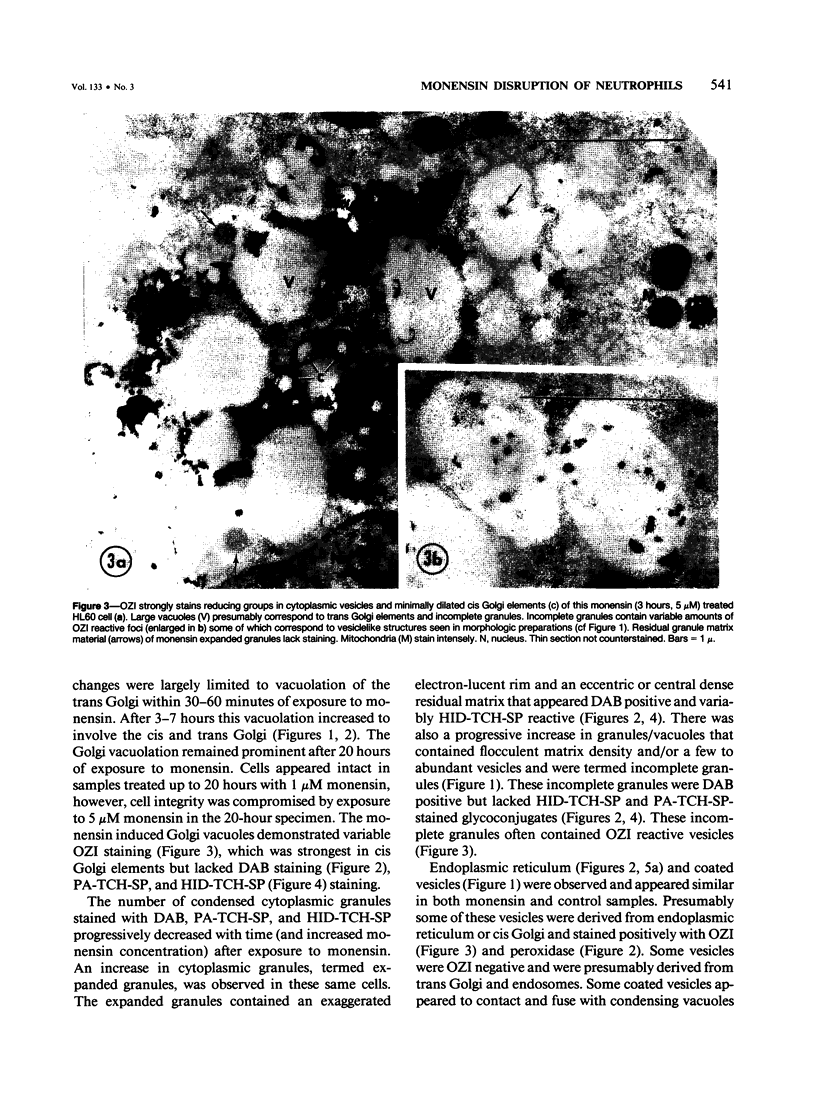
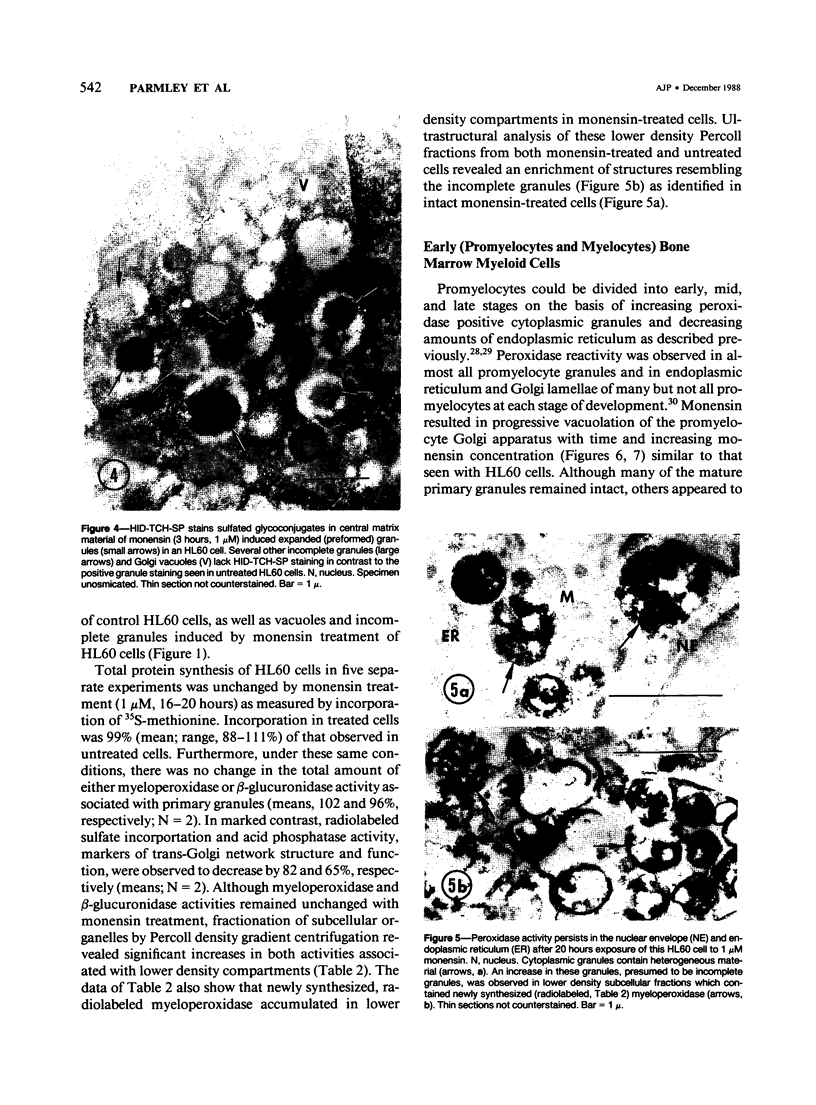
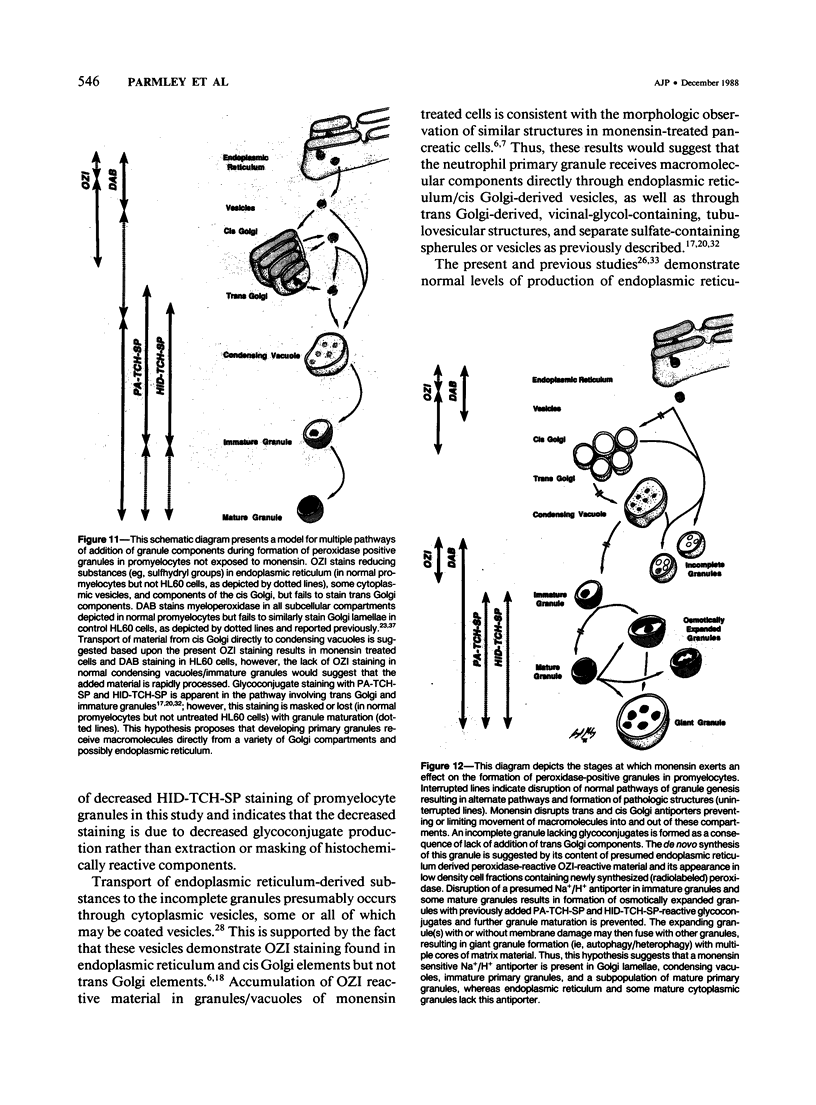
The Na+/H+ ionophore monensin (M) has been used widely to study intracellular pH gradients and acidic subcellular compartments. In the present study, cultured myeloid leukemia HL60 cells, directly sampled bone marrow cells, and peripheral blood neutrophils were exposed to 1-5 microM monensin for 0.5-20 hours. The effects were evaluated using ultrastructural, cytochemical, and biochemical methods. In HL60 cells and marrow promyelocytes treated with monensin, progressive vacuolation of the trans then the cis Golgi was observed. These vacuoles lacked diaminobenzidine (DAB) reactive peroxidase, high iron diamine (HID) reactive sulfated glycoconjugates, and periodate-thiocarbohydrazide-silver proteinate (PA-TCH-SP) reactive vicinal glycol containing complex carbohydrates, but some cis Golgi elements retained osmium zinc iodide reactive reducing groups. The number of normal intensely stained HID reactive granules decreased and an incomplete granule that was DAB-positive/HID-negative, PA-TCH-SP-negative with flocculent matrix density increased in frequency as a function of time and concentration of monensin. Treatment of HL60 cells with monensin markedly reduced 35SO4 incorporation but myeloperoxidase labeling and activity per cell remained constant, although it shifted to lower density granule fractions consistent with the persistent DAB staining of endoplasmic reticulum and synthesis of a DAB-positive, HID-negative granule in intact HL60 cells. The Golgi complex of monensin-treated myelocytes and segmented neutrophils was also vacuolated. A subpopulation of preformed primary granules in promyelocytes, myelocytes, and segmented neutrophils appeared to increase in size and peripheral or central electron lucency. These selective effects of monensin indicate that granule components may be packaged into DAB-positive organelles that are deficient in trans Golgi-derived elements (HID- and PA-TCH-SP-negative) and that some preformed primary granules contain a monensin sensitive Na+/H+ gradient.
Full text
PDF
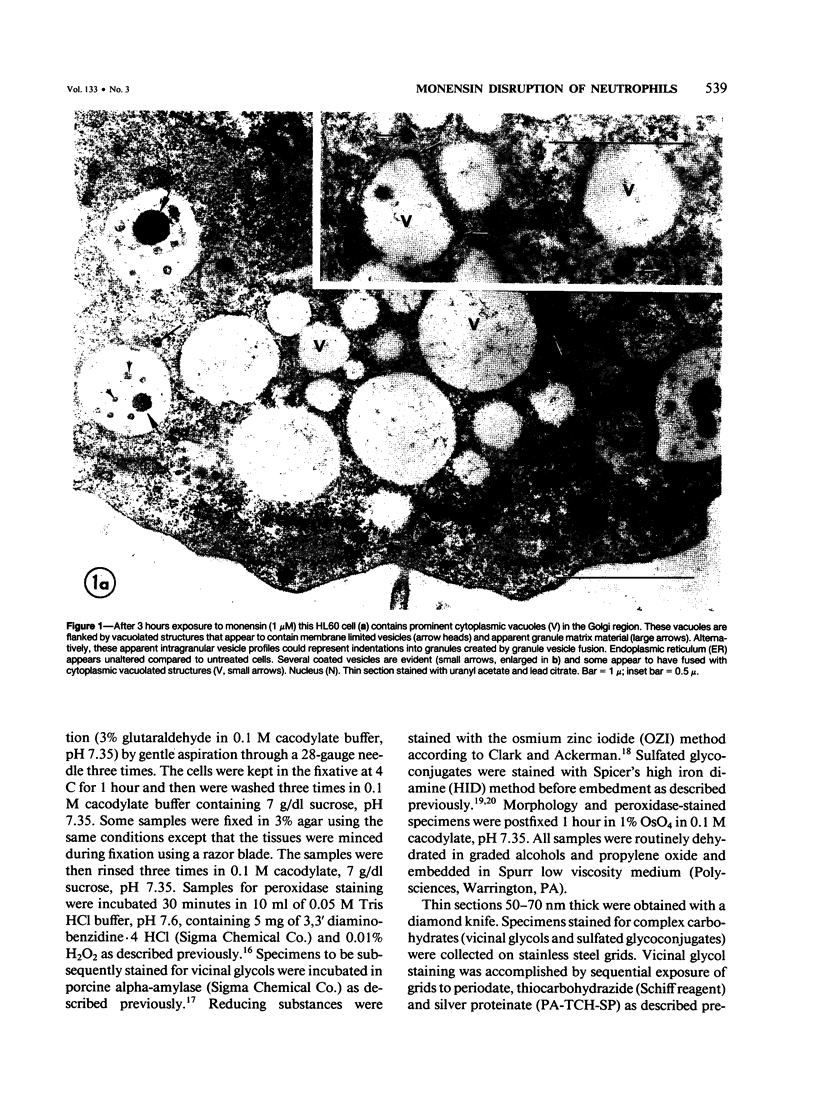





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman G. A., Clark M. A. Ultrastructural localization of peroxidase activity in normal human bone marrow cells. Z Zellforsch Mikrosk Anat. 1971;117(4):463–475. doi: 10.1007/BF00330708. [DOI] [PubMed] [Google Scholar]
- Ackerman G. A. The human neutrophilic myelocyte. A correlated phase and electron microscopic study. Z Zellforsch Mikrosk Anat. 1971;121(2):153–170. doi: 10.1007/BF00340669. [DOI] [PubMed] [Google Scholar]
- Ackerman G. A. The human neutrophilic promyelocyte. A correlated phase and electron microscopic study. Z Zellforsch Mikrosk Anat. 1971 Jul;118(4):467–481. doi: 10.1007/BF00324614. [DOI] [PubMed] [Google Scholar]
- Akin D. T., Kinkade J. M., Jr Evidence for the involvement of an acidic compartment in the processing of myeloperoxidase in human promyelocytic leukemia HL-60 cells. Arch Biochem Biophys. 1987 Jun;255(2):428–436. doi: 10.1016/0003-9861(87)90411-5. [DOI] [PubMed] [Google Scholar]
- Akin D. T., Kinkade J. M., Jr, Parmley R. T. Biochemical and ultrastructural effects of monensin on the processing, intracellular transport, and packaging of myeloperoxidase into low and high density compartments of human leukemia (HL-60) cells. Arch Biochem Biophys. 1987 Sep;257(2):451–463. doi: 10.1016/0003-9861(87)90590-x. [DOI] [PubMed] [Google Scholar]
- Akin D. T., Kinkade J. M., Jr Processing of a newly identified intermediate of human myeloperoxidase in isolated granules occurs at neutral pH. J Biol Chem. 1986 Jun 25;261(18):8370–8375. [PubMed] [Google Scholar]
- Bainton D. F. HL-60 cells have abnormal myeloperoxidase transport and packaging. Exp Hematol. 1988 Feb;16(2):150–158. [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brederoo P., van der Meulen J., Daems W. T. Ultrastructural localization of peroxidase activity in developing neutrophil granulocytes from human bone marrow. Histochemistry. 1986;84(4-6):445–453. doi: 10.1007/BF00482977. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Ackerman G. A. Osmium-zinc iodide reactivity in human blood and bone marrow cells. Anat Rec. 1971 May;170(1):81–95. doi: 10.1002/ar.1091700107. [DOI] [PubMed] [Google Scholar]
- Dunn W. B., Hardin J. H., Spicer S. S. Ultrastructural localization of myeloperoxidase in human neutrophil and rabbit heterophil and eosinophil leukocytes. Blood. 1968 Dec;32(6):935–944. [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fittschen C., Parmley R. T., Austin R. L., Crist W. M. Vicinal glycol-staining identifies secondary granules in human normal and Chédiak-Higashi neutrophils. Anat Rec. 1983 Mar;205(3):301–311. doi: 10.1002/ar.1092050307. [DOI] [PubMed] [Google Scholar]
- Forgac M., Cantley L. Characterization of the ATP-dependent proton pump of clathrin-coated vesicles. J Biol Chem. 1984 Jul 10;259(13):8101–8105. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Jesaitis R. K., Dahinden C. A., Chang C. M., Jesaitis A. J. Investigations on the role of Golgi-mediated, ligand-receptor processing in the activation of granulocytes by chemoattractants: differential effects of monensin. Biochim Biophys Acta. 1987 Mar 11;927(3):382–391. doi: 10.1016/0167-4889(87)90103-0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Hasilik A., von Figura K., Helmy S., Fishman J., Fine R. E., Kedersha N. L., Rome L. H. Lysosomal enzyme precursors in coated vesicles derived from the exocytic and endocytic pathways. J Cell Biol. 1987 Jun;104(6):1743–1748. doi: 10.1083/jcb.104.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M. Posttranslational processing of a human myeloid lysosomal protein, myeloperoxidase. Blood. 1987 Oct;70(4):1143–1150. [PubMed] [Google Scholar]
- Olsson I., Lantz M., Persson A. M., Arnljots K. Biosynthesis and processing of lactoferrin in bone marrow cells, a comparison with processing of myeloperoxidase. Blood. 1988 Feb;71(2):441–447. [PubMed] [Google Scholar]
- Orci L., Halban P., Amherdt M., Ravazzola M., Vassalli J. D., Perrelet A. A clathrin-coated, Golgi-related compartment of the insulin secreting cell accumulates proinsulin in the presence of monensin. Cell. 1984 Nov;39(1):39–47. doi: 10.1016/0092-8674(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986 Dec;103(6 Pt 1):2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley R. T., Akin D. T., Barton J. C., Gilbert C. S., Kinkade J. M., Jr Cytochemistry and ultrastructural morphometry of cultured HL60 myeloid leukemia cells. Cancer Res. 1987 Sep 15;47(18):4932–4940. [PubMed] [Google Scholar]
- Parmley R. T., Eguchi M., Spicer S. S., Alvarez C. J., Austin R. L. Ultrastructural cytochemistry and radioautography of complex carbohydrates in heterophil granulocytes from rabbit bone marrow. J Histochem Cytochem. 1980 Oct;28(10):1067–1080. doi: 10.1177/28.10.7419899. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Hurst R. E., Takagi M., Spicer S. S., Austin R. L. Glycosaminoglycans in human neutrophils and leukemic myeloblasts: ultrastructural, cytochemical, immunologic, and biochemical characterization. Blood. 1983 Feb;61(2):257–266. [PubMed] [Google Scholar]
- Parmley R. T., Rice W. G., Kinkade J. M., Jr, Gilbert C., Barton J. C. Peroxidase-containing microgranules in human neutrophils: physical, morphological, cytochemical, and secretory properties. Blood. 1987 Nov;70(5):1630–1638. [PubMed] [Google Scholar]
- Pember S. O., Kinkade J. M., Jr Differences in myeloperoxidase activity from neutrophilic polymorphonuclear leukocytes of differing density: relationship to selective exocytosis of distinct forms of the enzyme. Blood. 1983 Jun;61(6):1116–1124. [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Ganz T., Kinkade J. M., Jr, Selsted M. E., Lehrer R. I., Parmley R. T. Defensin-rich dense granules of human neutrophils. Blood. 1987 Sep;70(3):757–765. [PubMed] [Google Scholar]
- Rice W. G., Kinkade J. M., Jr, Parmley R. T. High resolution of heterogeneity among human neutrophil granules: physical, biochemical, and ultrastructural properties of isolated fractions. Blood. 1986 Aug;68(2):541–555. [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Sannes P. L., Spicer S. S., Katsuyama T. Ultrastructural localization of sulfated complex carbohydrates with a modified iron diamine procedure. J Histochem Cytochem. 1979 Jul;27(7):1108–1111. doi: 10.1177/27.7.89157. [DOI] [PubMed] [Google Scholar]
- Scherman D., Nordmann J., Henry J. P. Existence of an adenosine 5'-triphosphate dependent proton translocase in bovine neurosecretory granule membrane. Biochemistry. 1982 Feb 16;21(4):687–694. doi: 10.1021/bi00533a016. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. I. Sodium fluxes. J Biol Chem. 1985 Oct 25;260(24):13237–13247. [PubMed] [Google Scholar]
- Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J Biol Chem. 1985 Oct 25;260(24):13248–13255. [PubMed] [Google Scholar]
- Wright J., Schwartz J. H., Olson R., Kosowsky J. M., Tauber A. I. Proton secretion by the sodium/hydrogen ion antiporter in the human neutrophil. J Clin Invest. 1986 Mar;77(3):782–788. doi: 10.1172/JCI112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Schneider D. L. The bioenergetics of Golgi apparatus function: evidence for an ATP-dependent proton pump. Biochem Biophys Res Commun. 1983 Jul 29;114(2):620–625. doi: 10.1016/0006-291x(83)90825-2. [DOI] [PubMed] [Google Scholar]