Abstract
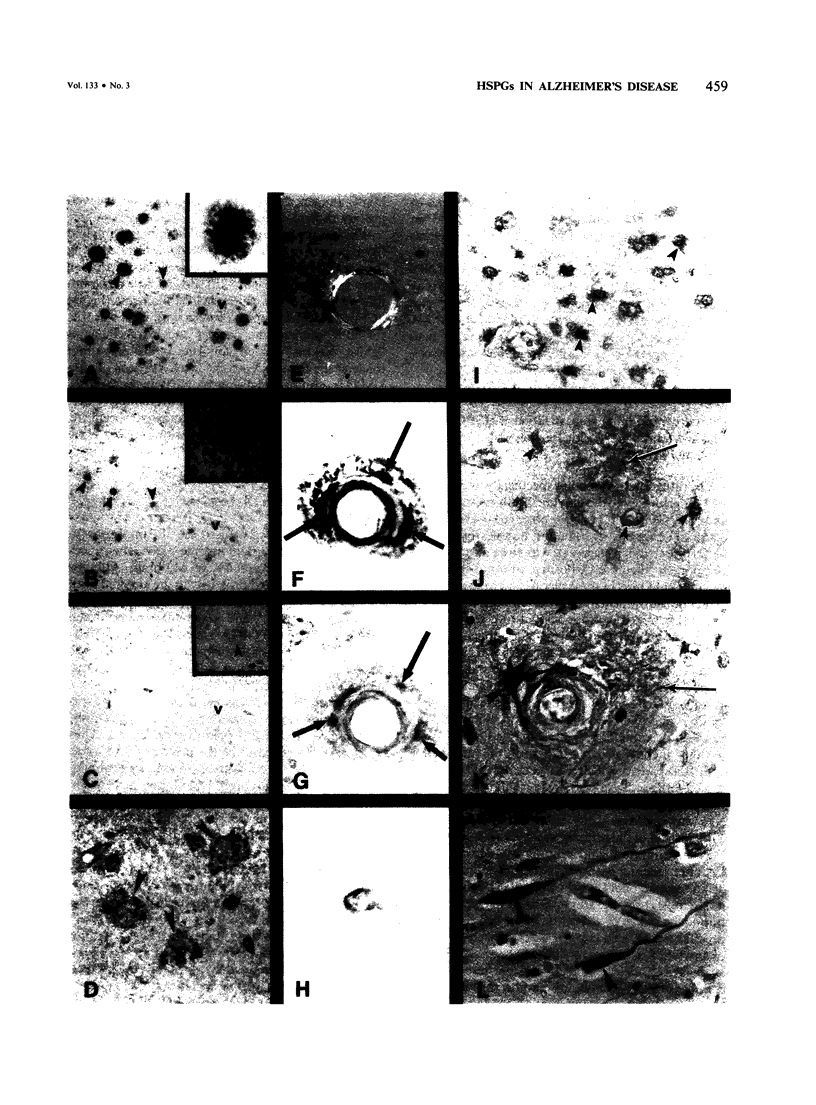
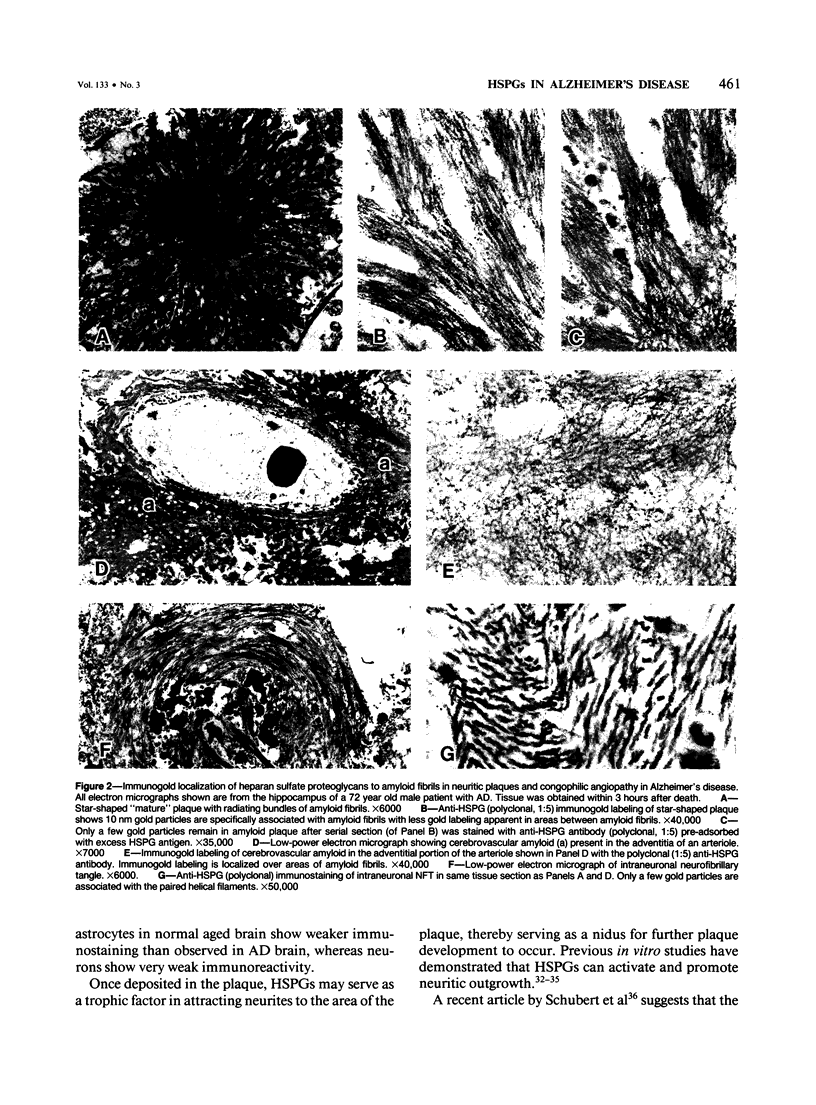
Two immunocytochemical probes were used to specifically identify and localize heparan sulphate proteoglycans (HSPGs) in 17 cases of Alzheimer's disease (AD). A monoclonal (HK-102) and an affinity-purified polyclonal antibody, each recognizing specific domains on the protein core of a basement membrane-derived HSPG, localized HSPGs to the amyloid fibrils present in neuritic plaques (NPs) and congophilic angiopathy (CA) in the brains of Alzheimer's patients, with weak to no immunostaining in neurofibrillary tangles from the same tissues. HSPGs were also demonstrated in "primitive plaques," suggesting that their accumulation takes place during early stages of plaque development. Immunolocalization of HSPGs to subsets of astrocytes and neuronal cells, particularly those in close proximity to NPs and CA, suggested possible involvement of these two cell types in deposition of HS-PGs into the amyloidotic lesions. The current study not only identifies a new component (HSPGs) present in the amyloid deposits of NPs and CA but also suggests that astrocytes, neurons, or both may be involved in its deposition at these sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Chernoff E. A. The role of endogenous heparan sulfate proteoglycan in adhesion and neurite outgrowth from dorsal root ganglia. Tissue Cell. 1988;20(2):165–178. doi: 10.1016/0040-8166(88)90039-0. [DOI] [PubMed] [Google Scholar]
- David S. Neurite outgrowth from mammalian CNS neurons on astrocytes in vitro may not be mediated primarily by laminin. J Neurocytol. 1988 Feb;17(1):131–144. doi: 10.1007/BF01735385. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Leblond C. P. Immunogold quantitation of laminin, type IV collagen, and heparan sulfate proteoglycan in a variety of basement membranes. J Histochem Cytochem. 1988 Mar;36(3):271–283. doi: 10.1177/36.3.2963856. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. A., Armstrong D. M., Terry R. D. An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging. 1987 Jan-Feb;8(1):1–6. doi: 10.1016/0197-4580(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh M. C., Probst A., Ulrich J., Kahn J., Anderton B. H. Alzheimer neurofibrillary tangles contain phosphorylated and hidden neurofilament epitopes. J Neurol Neurosurg Psychiatry. 1986 Nov;49(11):1213–1220. doi: 10.1136/jnnp.49.11.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kato M., Koike Y., Ito Y., Suzuki S., Kimata K. Multiple forms of heparan sulfate proteoglycans in the Engelbreth-Holm-Swarm mouse tumor. The occurrence of high density forms bearing both heparan sulfate and chondroitin sulfate side chains. J Biol Chem. 1987 May 25;262(15):7180–7188. [PubMed] [Google Scholar]
- Kato M., Koike Y., Suzuki S., Kimata K. Basement membrane proteoglycan in various tissues: characterization using monoclonal antibodies to the Engelbreth-Holm-Swarm mouse tumor low density heparan sulfate proteoglycan. J Cell Biol. 1988 Jun;106(6):2203–2210. doi: 10.1083/jcb.106.6.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Brenchley P. E., Anderson J. C., Dickinson M. A., Horigan E. A., Hassell J. R. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988 Mar;106(3):963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar H., Wight T. N. Colloidal gold immunostaining on deplasticized ultra-thin sections. J Histochem Cytochem. 1988 Nov;36(11):1387–1395. doi: 10.1177/36.11.2844888. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Lewis D. A., Campbell M. J., Huntley G. W., Benson D. L., Bouras C. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer's disease. Brain Res. 1987 Jul 28;416(2):331–336. doi: 10.1016/0006-8993(87)90914-0. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Stephens C., Anastassiades T. Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Lab Invest. 1987 Jun;56(6):665–675. [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest. 1985 Jul;53(1):37–44. [PubMed] [Google Scholar]
- Snow A. D., Willmer J. P., Kisilevsky R. Sulfated glycosaminoglycans in Alzheimer's disease. Hum Pathol. 1987 May;18(5):506–510. doi: 10.1016/s0046-8177(87)80036-9. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest. 1987 Jan;56(1):120–123. [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wujek J. R., Akeson R. A. Extracellular matrix derived from astrocytes stimulates neuritic outgrowth from PC12 cells in vitro. Brain Res. 1987 Jul;431(1):87–97. doi: 10.1016/0165-3806(87)90198-2. [DOI] [PubMed] [Google Scholar]