Abstract
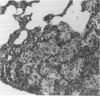
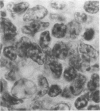
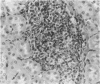
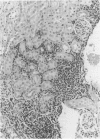
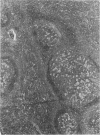
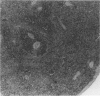
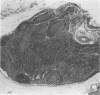
Experimental infection with Herpesvirus sylvilagus produces clinical and histopathologic changes in its natural host, the cottontail rabbit (Sylvilagus floridanus), similar to those observed in humans acutely infected with Epstein-Barr virus (EBV). Twenty-seven seronegative cottontail rabbits were infected with Herpesvirus sylvilagus and all developed antibodies within 10 days. Neutralizing antibody was detected as early as 7 days after infection. Virus was isolated from blood mononuclear cells, spleen, bone marrow, thymus, lymph nodes, kidneys, lung, and liver as early as 3 days after infection. Infected animals showed leucocytosis, monocytosis, and lymphocytosis with the appearance of atypical lymphocytes. Peripheral blood abnormalities peaked at 10-14 days after infection, and returned to normal by 28 days after infection, with the exception of atypical lymphocytosis that persisted in some animals for more than 2 years after experimental infection. More severe histopathologic changes were seen in virus-infected juvenile rabbits than adult rabbits; these changes included viral myocarditis, interstitial pneumonia, and lymphocytic myositis. Reactive hyperplasia and subsequent lymphocytic depletion of spleen and lymph nodes were reminiscent of that seen in virus-associated hemophagocytosis syndrome. Prominent lymphoid hyperplasia of many nonlymphoid organs, most notably the kidney and lungs, was observed. The development of these lymphoproliferative lesions and other lymphoid changes during H. sylvilagus infection suggest that this system may be a model to study similar lesions induced by EBV infection in humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohrs R., Rouhandeh H. Herpesvirus sylvilagus I. Polypeptides of virions and nucleocapsids. J Virol. 1982 Mar;41(3):1063–1072. doi: 10.1128/jvi.41.3.1063-1072.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Rabin H., Ball G., Rickinson A. B., Jarvis J., Meléndez L. V. Pilot experiments with EB virus in owl monkeys (Aotus trivirgatus). II. EB virus in a cell line from an animal with reticuloproliferative disease. Int J Cancer. 1973 Sep 15;12(2):319–332. doi: 10.1002/ijc.2910120203. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Hinze H. C. Morphological studies on Herpesvirus sylvilagus in rabbit kidney cell cultures. Cancer Res. 1972 Jun;32(6):1340–1350. [PubMed] [Google Scholar]
- Hinze H. C., Chipman P. J. Role of herpesviruses in malignant lymphoma in rabbits. Fed Proc. 1972 Nov-Dec;31(6):1639–1642. [PubMed] [Google Scholar]
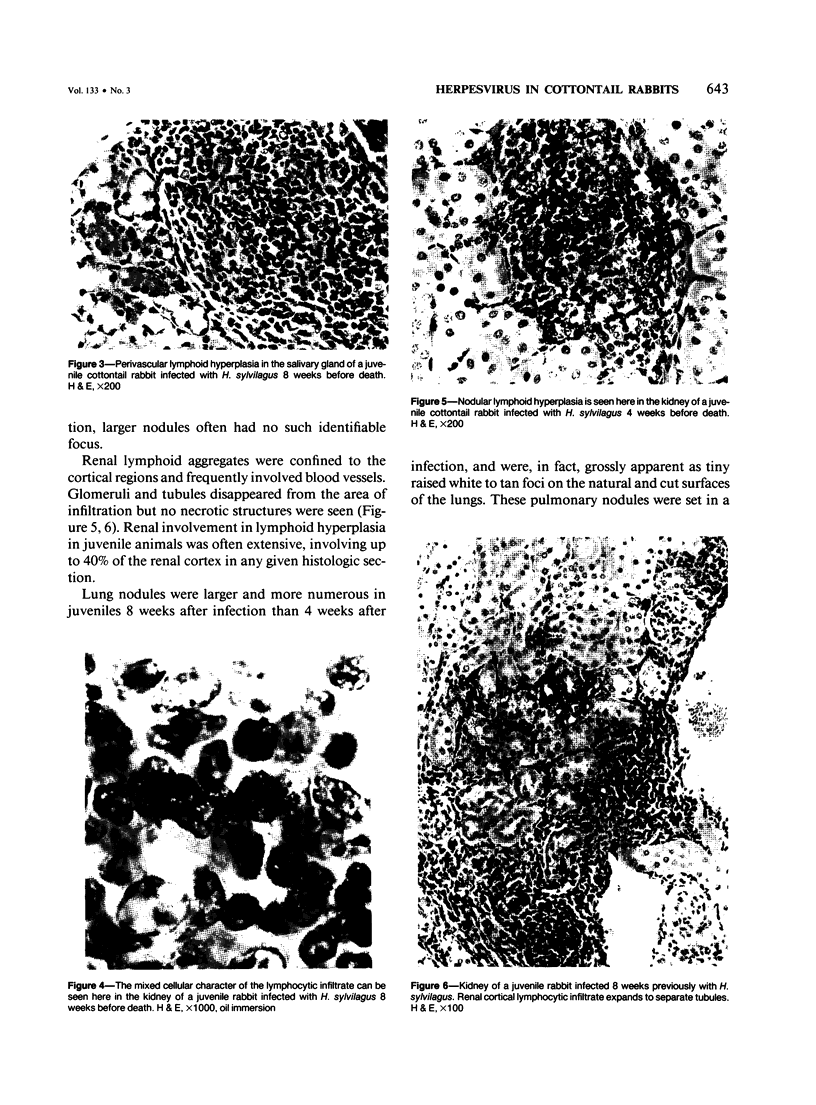
- Hinze H. C. Induction of lymphoid hyperplasia and lymphoma-like disease in rabbits by Herpesvirus sylvilagus. Int J Cancer. 1971 Nov 15;8(3):514–522. doi: 10.1002/ijc.2910080320. [DOI] [PubMed] [Google Scholar]
- Hinze H. C. New member of the herpesvirus group isolated from wild cottontail rabbits. Infect Immun. 1971 Feb;3(2):350–354. doi: 10.1128/iai.3.2.350-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H. C., Wegner D. L. Oncogenicity of rabbit herpesvirus. Cancer Res. 1973 Jun;33(6):1434–1435. [PubMed] [Google Scholar]
- Ley K. D., Burger D. Cell-associated nature of cottontail rabbit herpesvirus in vitro. Appl Microbiol. 1970 Mar;19(3):549–550. doi: 10.1128/am.19.3.549-550.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medveczky P., Kramp W. J., Sullivan J. L. Circular Herpesvirus sylvilagus DNA in spleen cells of experimentally infected cottontail rabbits. J Virol. 1984 Nov;52(2):711–714. doi: 10.1128/jvi.52.2.711-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
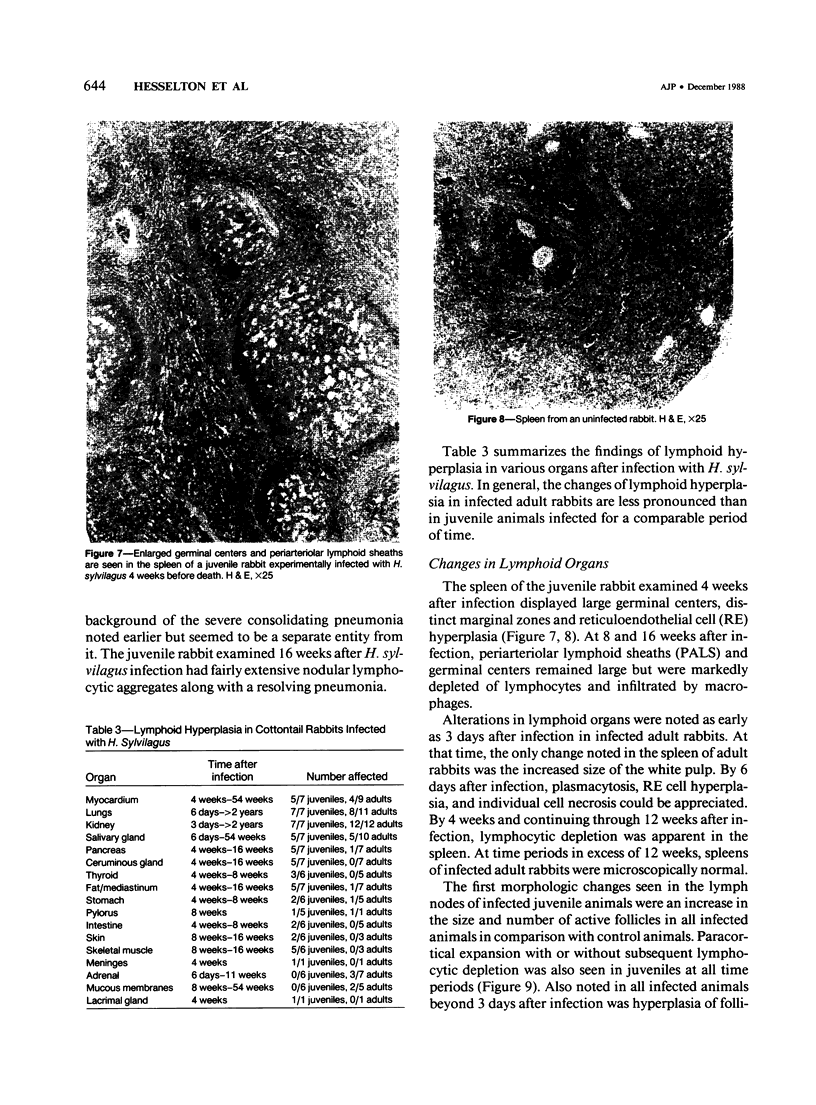
- Spieker J. O., Yuill T. M. Herpesvirus sylvilagus in cottontail rabbits: antibody prevalence and flea burden relationships. J Wildl Dis. 1976 Jul;12(3):310–314. doi: 10.7589/0090-3558-12.3.310. [DOI] [PubMed] [Google Scholar]
- Spieker J. O., Yuill T. M. Herpesvirus sylvilagus in cottontail rabbits: evidence of shedding but not transplacental transmission. J Wildl Dis. 1977 Jan;13(1):85–89. doi: 10.7589/0090-3558-13.1.85. [DOI] [PubMed] [Google Scholar]
- Sugden B. Epstein-Barr virus: a human pathogen inducing lymphoproliferation in vivo and in vitro. Rev Infect Dis. 1982 Sep-Oct;4(5):1048–1061. doi: 10.1093/clinids/4.5.1048. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Silver S., Nonoyama M. Biochemical evidence of the nonintegrated status of Marek's disease virus DNA in virus-transformed lymphoblastoid cells of chicken. Virology. 1978 Jul 1;88(1):19–24. doi: 10.1016/0042-6822(78)90105-8. [DOI] [PubMed] [Google Scholar]
- Tomkinson B. E., Wagner D. K., Nelson D. L., Sullivan J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987 Dec 1;139(11):3802–3807. [PubMed] [Google Scholar]
- Wegner D. L., Hinze H. C. Virus--host-cell relationship of Herpesvirus sylvilagus with cottontail rabbit leukocytes. Int J Cancer. 1974 Nov 15;14(5):567–575. doi: 10.1002/ijc.2910140502. [DOI] [PubMed] [Google Scholar]