Abstract
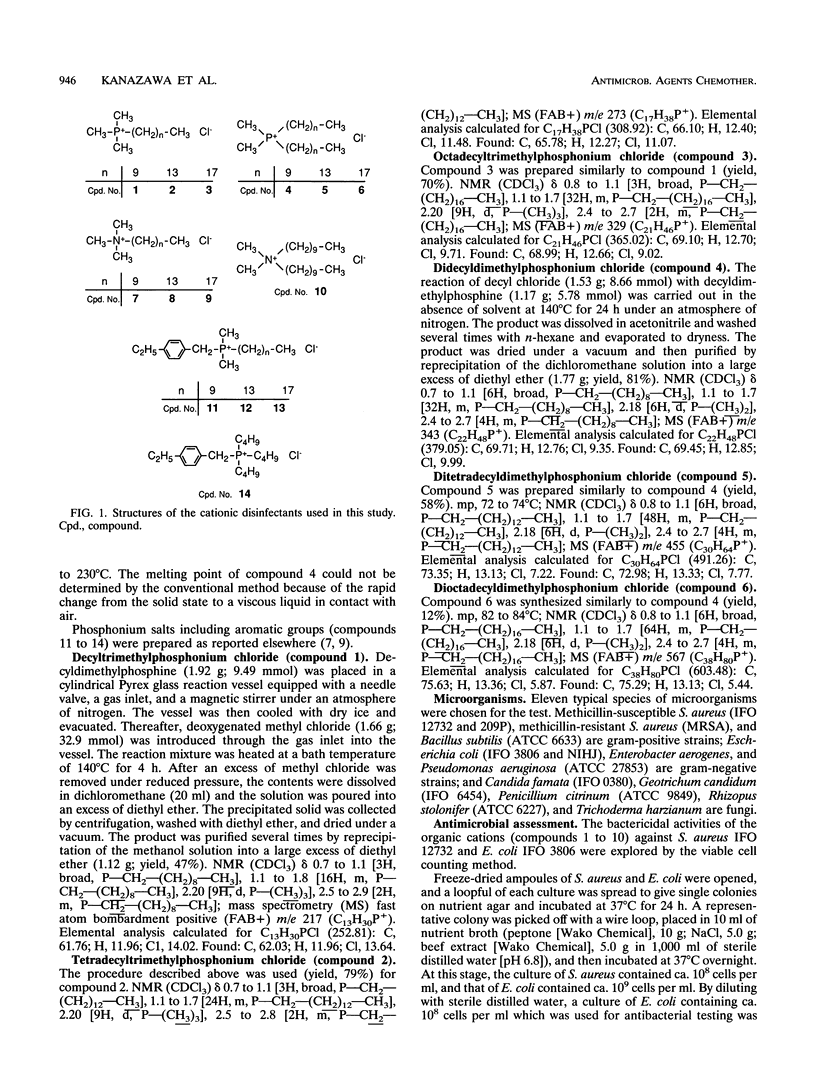
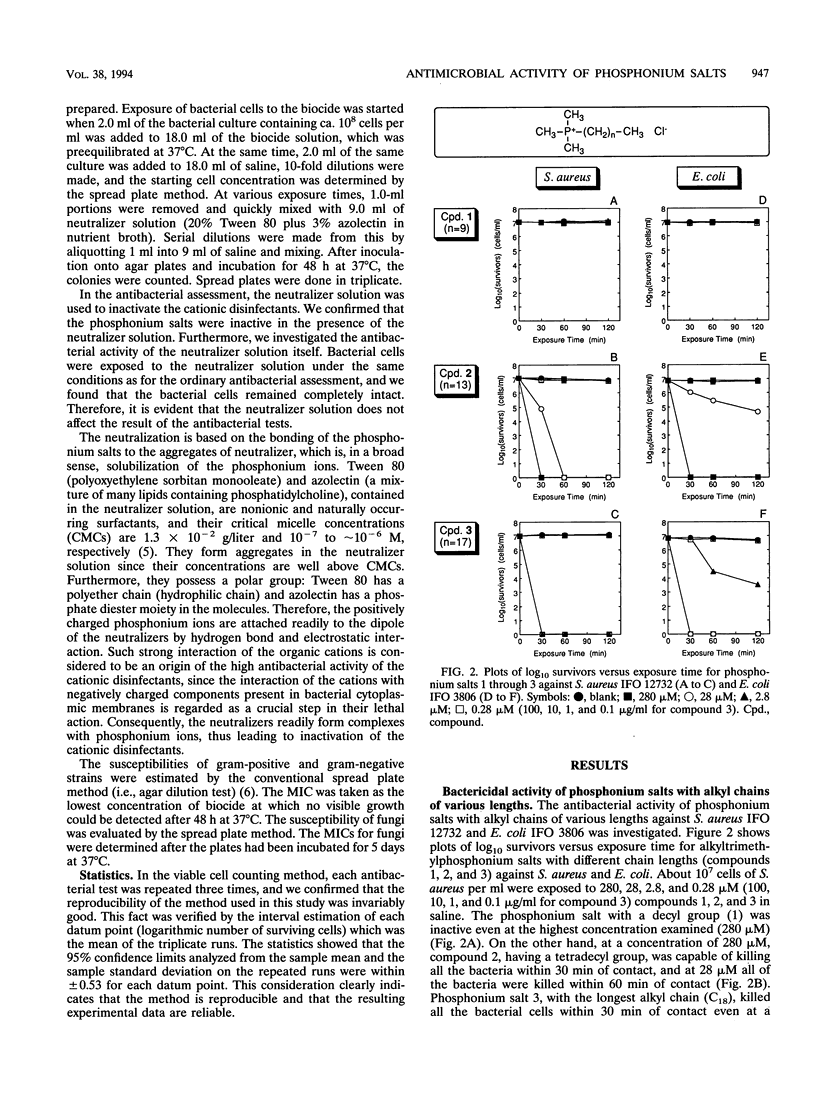
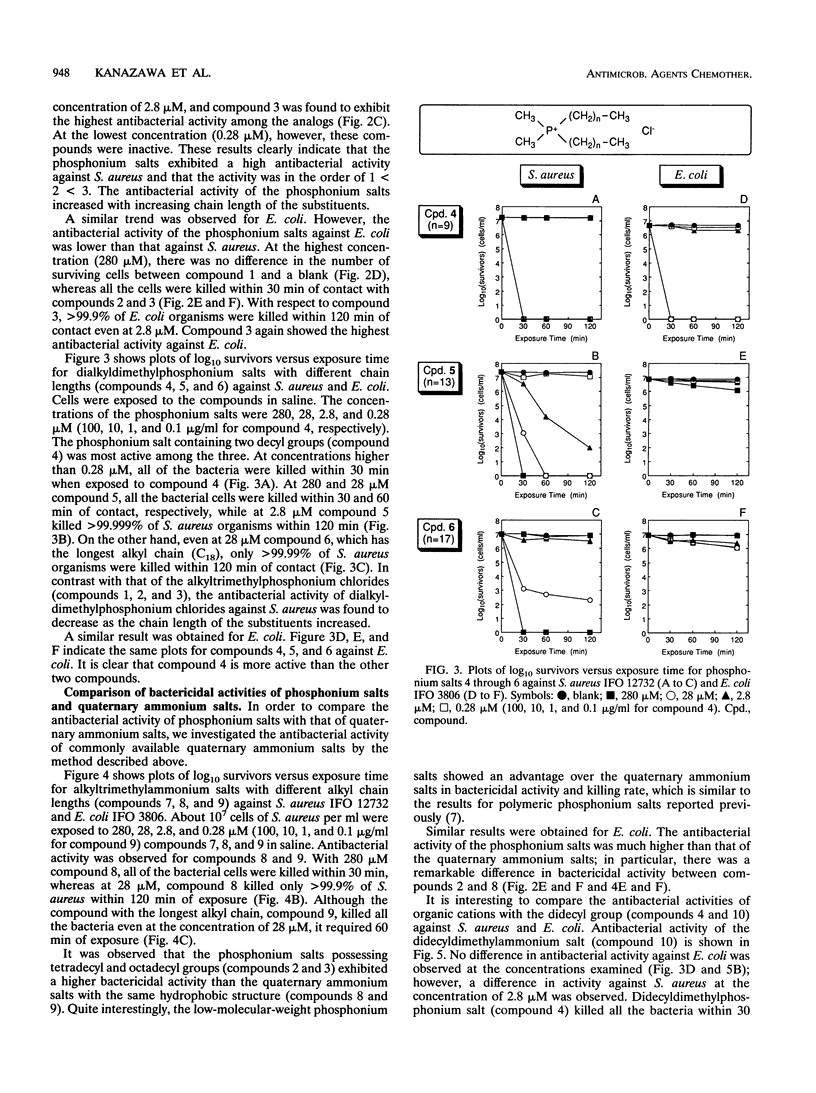
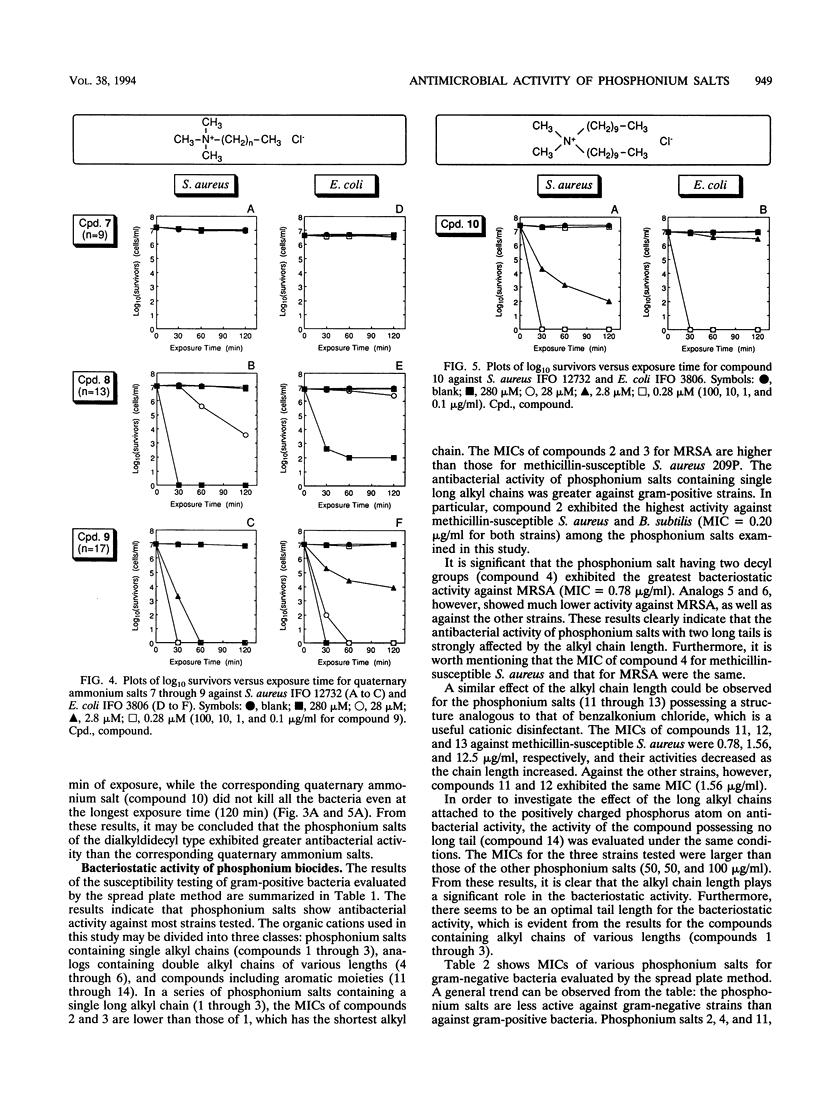
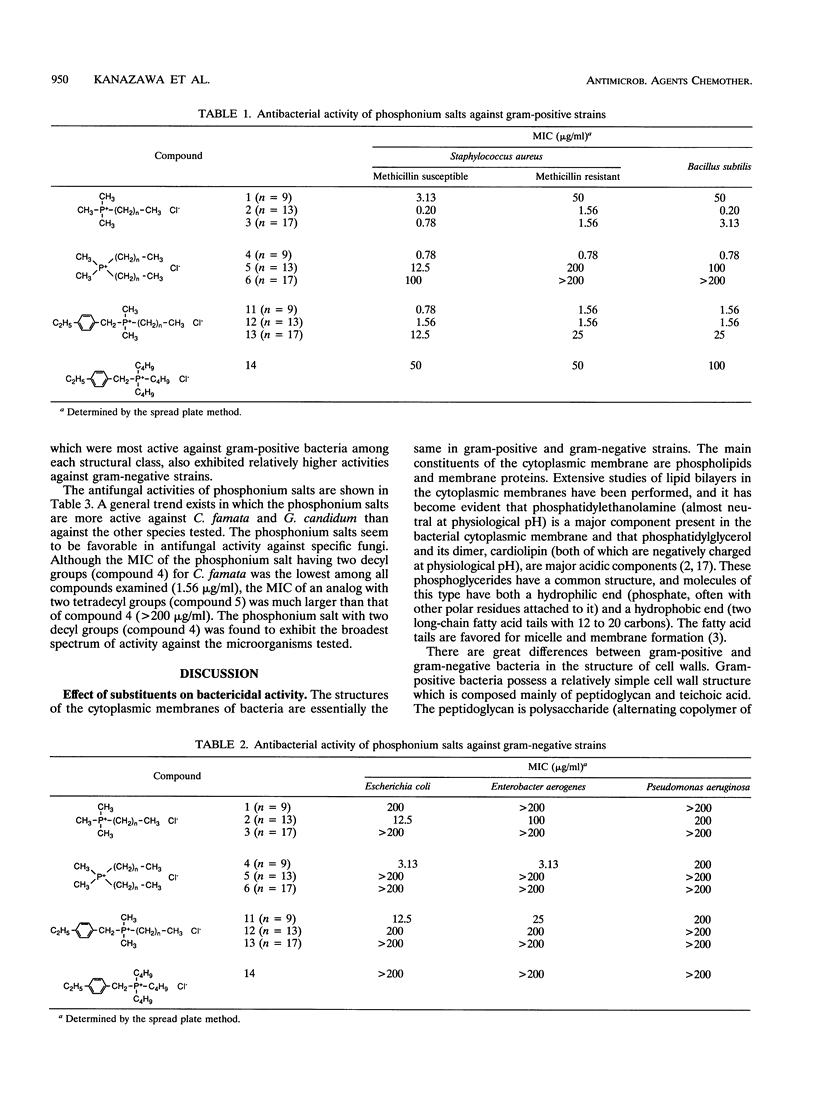
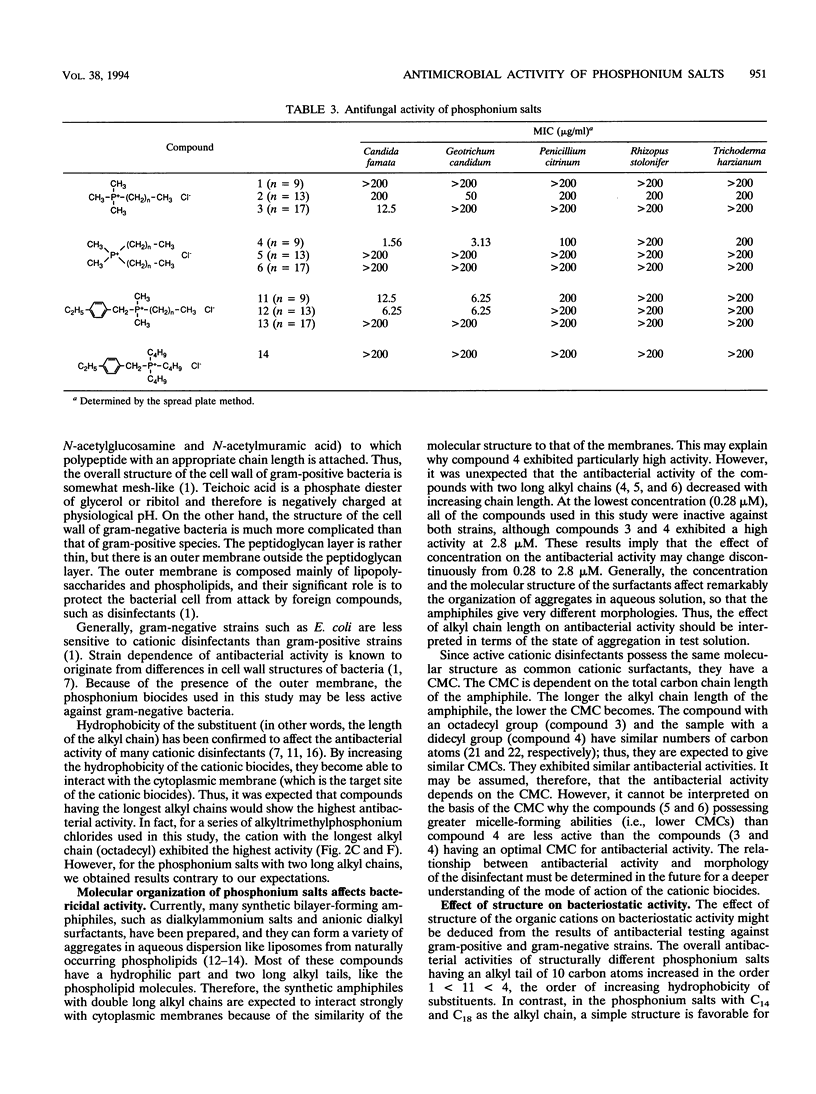
Various phosphonium salts possessing single or double alkyl chains of various lengths (C10 to C18) were prepared as cationic biocides, and their antimicrobial activities against 11 typical strains of microorganisms including methicillin-resistant Staphylococcus aureus (MRSA) were evaluated. The phosphonium salts with long alkyl chains were found to show high levels of antimicrobial activity. Their activities depended strongly on the molecular structure, and a correlation between antimicrobial activity and molecular structure was observed. In the alkyltrimethylphosphonium salts, the bactericidal activity against S. aureus and Escherichia coli increased with increasing alkyl chain length, and the compound with the longest alkyl chain (C18) killed all the bacterial cells (ca. 10(7) cells per ml) within 30 min of contact at concentrations of 2.8 and 28 microM, respectively. In contrast, the bactericidal activity of dialkyldimethylphosphonium salts was found to decrease as the chain length of the substituents increased. It is significant that the phosphonium biocide containing double decyl groups exhibited the broadest spectrum of activity against microorganisms tested and showed the greatest bacteriostatic activity against MRSA (MIC = 0.78 micrograms/ml). Furthermore, we systematically investigated differences in bactericidal activity between the phosphonium salts and commonly available ammonium salts with the same hydrophobic structure. It was observed that the phosphonium salts showed an advantage over the corresponding ammonium salts in bactericidal activity and killing rate. For example, tetradecyltrimethyl- and didecyldimethylphosphonium chlorides killed all S. aureus organisms (ca. 10(7) cells per ml) within 60 and 30 min of exposure at 28 and 2.8 microM, respectively, while tetradecyltrimethyl- and didecyldimethylammonium chlorides which are representative of the existing cationic disinfectants did not kill all the bacteria even at the longest exposure time (120 min).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Cheng K. J. The role of the bacterial cell envelope in antibiotic resistance. J Antimicrob Chemother. 1975 Dec;1(4):363–377. doi: 10.1093/jac/1.4.363. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsmore R. Biocidal control of legionellae. Isr J Med Sci. 1986 Sep;22(9):647–654. [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


