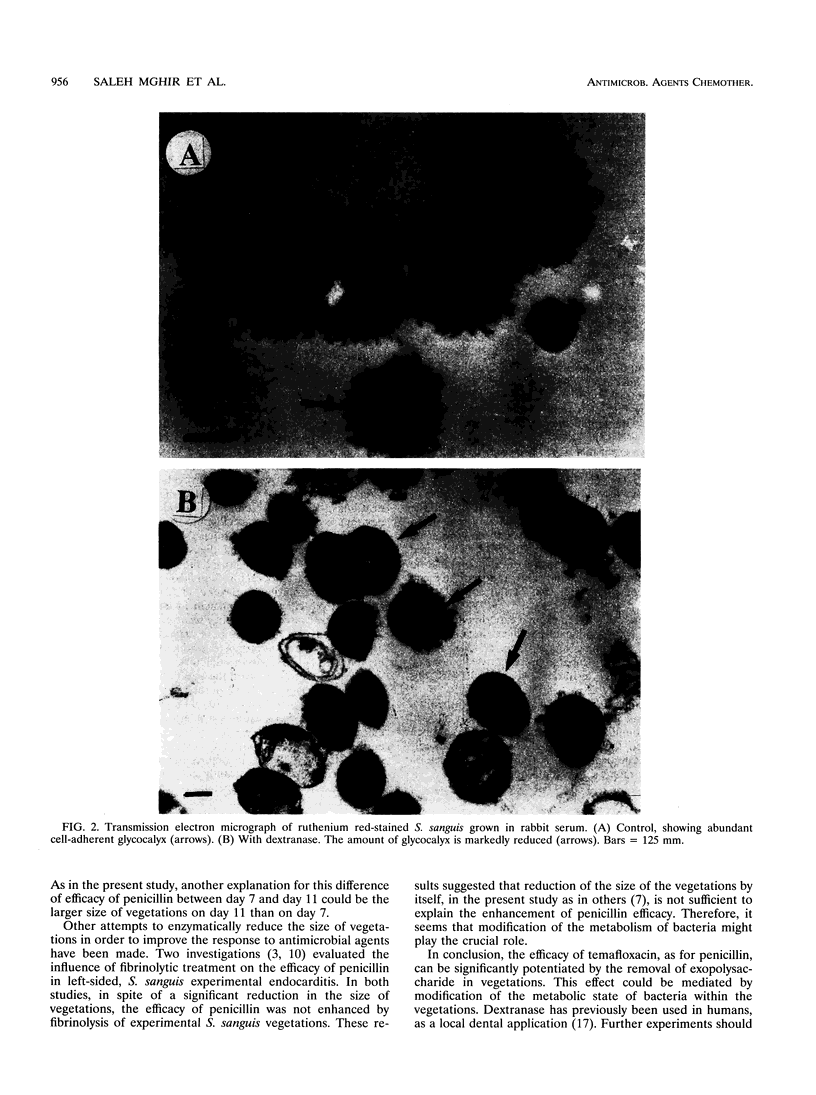
Abstract
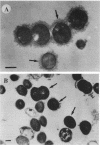
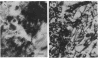
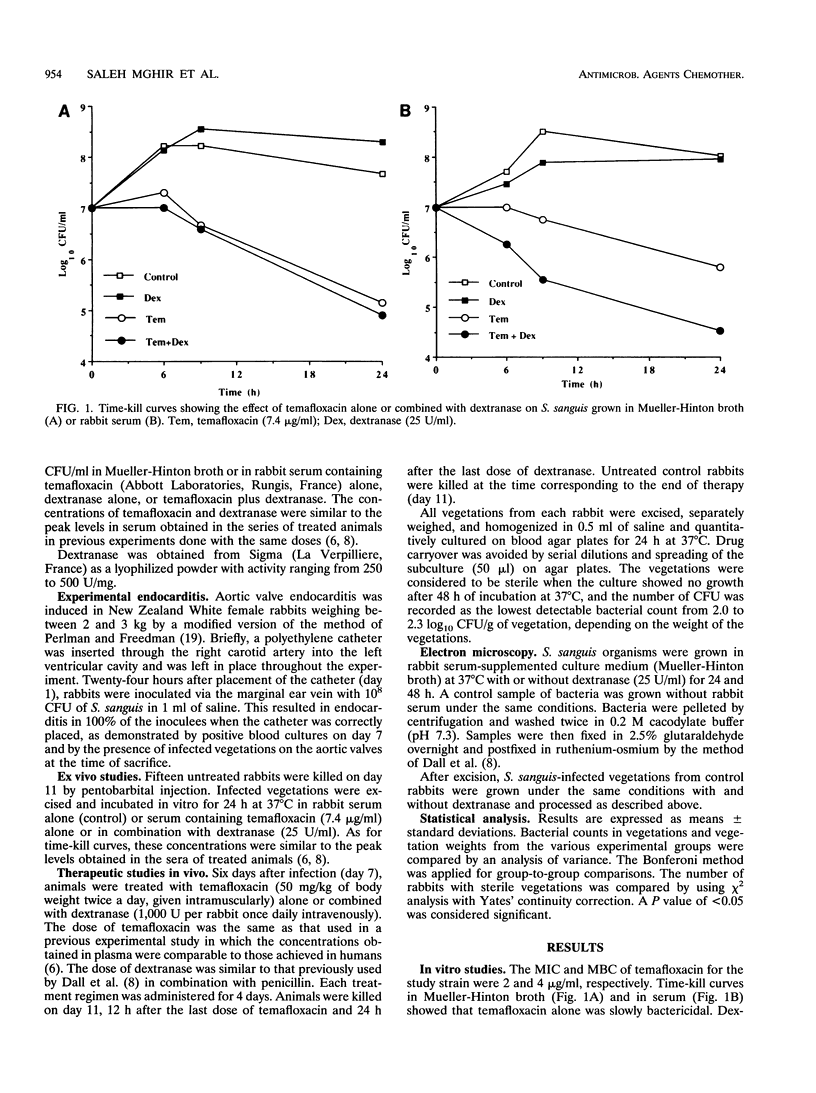
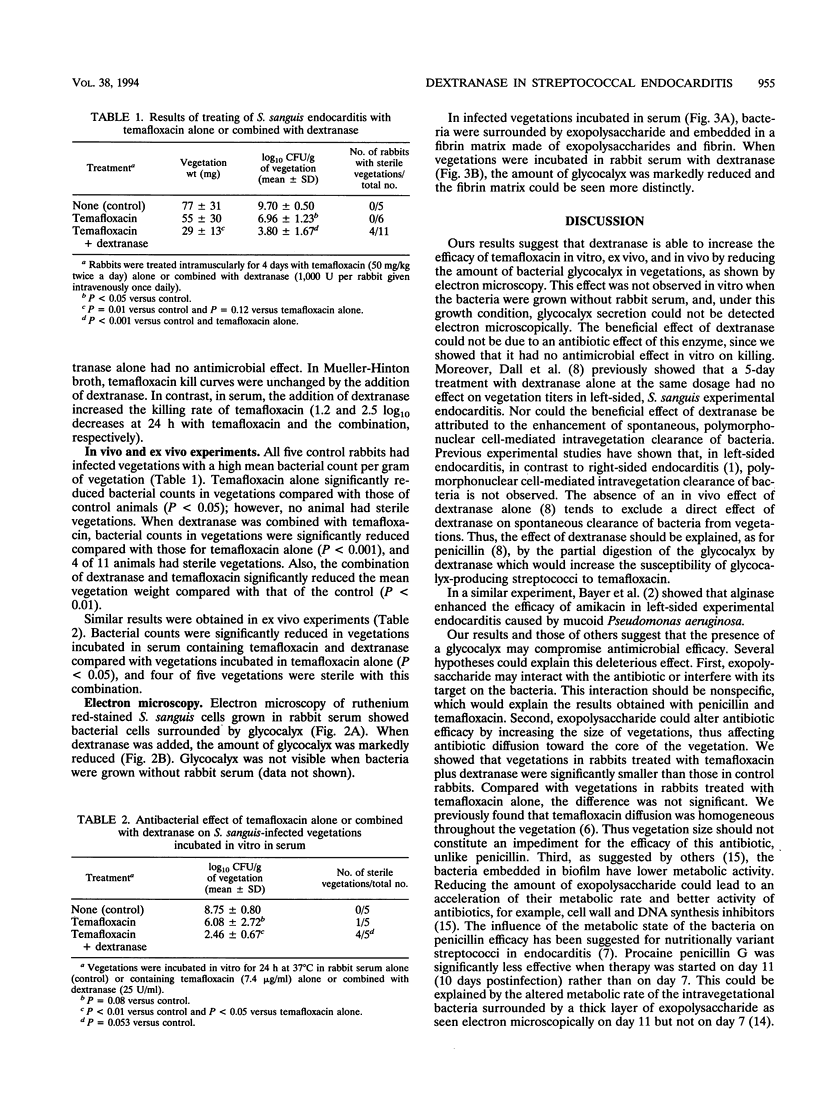
In endocarditis, exopolysaccharide production by viridans streptococci has been associated with delayed antimicrobial efficacy in cardiac vegetations. We compared the efficacies of temafloxacin alone and in combination with dextranase, an enzyme capable of hydrolyzing 20 to 90% of the bacterial glycocalyx, in a rabbit model of endocarditis. In in vivo experiments, rabbits were infected intravenously with 10(8) Streptococcus sanguis organisms and were treated 6 days later with temafloxacin (50 mg/kg of body weight intramuscularly twice a day) alone or combined with dextranase (1,000 U per rabbit per day intravenously). After 4 days of treatment (day 11), the animals were sacrificed and vegetations were quantitatively cultured. For ex vivo experiments, rabbits were infected as stated above and, on day 11, vegetations were excised aseptically and incubated in vitro in rabbit serum alone (control) or with temafloxacin or temafloxacin plus dextranase at concentrations similar to peak levels in plasma. In vitro, dextranase alone had no antimicrobial effect. In vivo and ex vivo, temafloxacin combined with dextranase was more effective than temafloxacin alone (P < 0.05). Our results suggest that dextranase is able to increase the effects of temafloxacin by reducing the amount of bacterial glycocalyx in infected vegetations, as confirmed in vitro by electron microscopy showing a markedly reduced amount of glycocalyx and a more clearly visible fibrin matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. S., Park S., Ramos M. C., Nast C. C., Eftekhar F., Schiller N. L. Effects of alginase on the natural history and antibiotic therapy of experimental endocarditis caused by mucoid Pseudomonas aeruginosa. Infect Immun. 1992 Oct;60(10):3979–3985. doi: 10.1128/iai.60.10.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Yih J., Chiu C. Y., Nast C. C. Pathogenic effects of monocytopenia, granulocytopenia and dexamethasone on the course of experimental Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1989;35(4):278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- Buiting A. G., Thompson J., Emeis J. J., Mattie H., Brommer E. J., van Furth R. Effect of tissue-type plasminogen activator (t-PA) on the treatment with benzylpenicillin of rabbits with experimental Streptococcus sanguis endocarditis. J Infect Dis. 1989 Apr;159(4):780–784. doi: 10.1093/infdis/159.4.780. [DOI] [PubMed] [Google Scholar]
- Costerton J. W. The etiology and persistence of cryptic bacterial infections: a hypothesis. Rev Infect Dis. 1984 Sep-Oct;6 (Suppl 3):S608–S616. doi: 10.1093/clinids/6.supplement_3.s608. [DOI] [PubMed] [Google Scholar]
- Cremieux A. C., Maziere B., Vallois J. M., Ottaviani M., Azancot A., Raffoul H., Bouvet A., Pocidalo J. J., Carbon C. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis. 1989 May;159(5):938–944. doi: 10.1093/infdis/159.5.938. [DOI] [PubMed] [Google Scholar]
- Cremieux A. C., Saleh-Mghir A., Vallois J. M., Maziere B., Muffat-Joly M., Devine C., Bouvet A., Pocidalo J. J., Carbon C. Efficacy of temafloxacin in experimental Streptococcus adjacens endocarditis and autoradiographic diffusion pattern of [14C]temafloxacin in cardiac vegetations. Antimicrob Agents Chemother. 1992 Oct;36(10):2216–2221. doi: 10.1128/aac.36.10.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémieux A. C., Saleh-Mghir A., Vallois J. M., Muffat-Joly M., Devine C., Pocidalo J. J., Carbon C. Influence of the pre-treatment duration of infection on the efficacies of various antibiotic regimens in experimental streptococcal endocarditis. J Antimicrob Chemother. 1993 Dec;32(6):843–852. doi: 10.1093/jac/32.6.843. [DOI] [PubMed] [Google Scholar]
- Dall L., Barnes W. G., Lane J. W., Mills J. Enzymatic modification of glycocalyx in the treatment of experimental endocarditis due to viridans streptococci. J Infect Dis. 1987 Nov;156(5):736–740. doi: 10.1093/infdis/156.5.736. [DOI] [PubMed] [Google Scholar]
- Dall L., Herndon B. Quantitative assay of glycocalyx produced by viridans group streptococci that cause endocarditis. J Clin Microbiol. 1989 Sep;27(9):2039–2041. doi: 10.1128/jcm.27.9.2039-2041.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar H. A., Jones M. R., Barnes W. S., Griffin S. G. Fibrinolytic therapy in bacterial endocarditis: experimental studies in dogs. Eur Heart J. 1986 Jun;7(6):520–527. doi: 10.1093/oxfordjournals.eurheartj.a062100. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972 Feb;53(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Kaplan M. H., Clogston A. G. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990 Jan;161(1):37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- Frehel C., Hellio R., Cremieux A. C., Contrepois A., Bouvet A. Nutritionally variant streptococci develop ultrastructural abnormalities during experimental endocarditis. Microb Pathog. 1988 Apr;4(4):247–255. doi: 10.1016/0882-4010(88)90085-x. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Collier P. J., Brown M. R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990 Oct;34(10):1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobene R. R. 2. A clinical study of the effect of dextranase on human dental plaque. J Am Dent Assoc. 1971 Jan;82(1):132–135. doi: 10.14219/jada.archive.1971.0014. [DOI] [PubMed] [Google Scholar]
- Mills J., Pulliam L., Dall L., Marzouk J., Wilson W., Costerton J. W. Exopolysaccharide production by viridans streptococci in experimental endocarditis. Infect Immun. 1984 Jan;43(1):359–367. doi: 10.1128/iai.43.1.359-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman B. B., Freedman L. R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971 Oct;44(2):206–213. [PMC free article] [PubMed] [Google Scholar]
- Pulliam L., Dall L., Inokuchi S., Wilson W., Hadley W. K., Mills J. Effects of exopolysaccharide production by viridans streptococci on penicillin therapy of experimental endocarditis. J Infect Dis. 1985 Jan;151(1):153–156. doi: 10.1093/infdis/151.1.153. [DOI] [PubMed] [Google Scholar]