Abstract
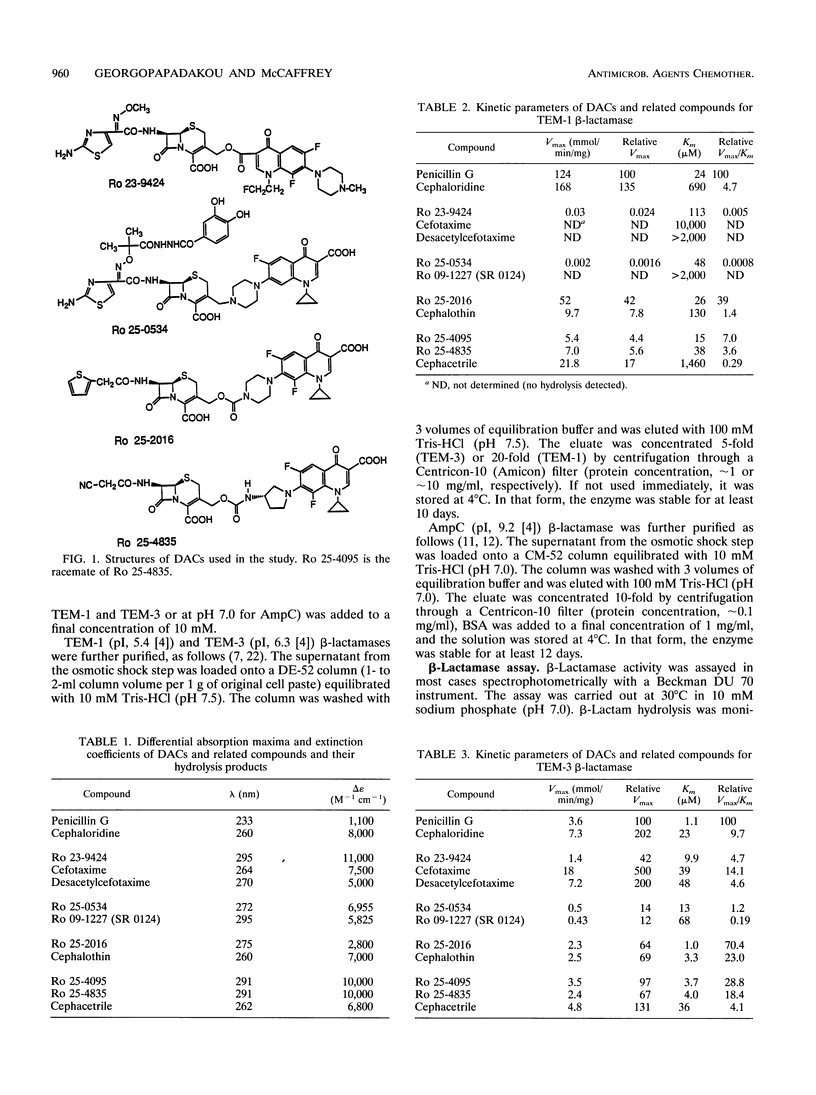
The beta-lactam hydrolysis of five cephalosporin 3'-quinolones (dual-action cephalosporins) by three gram-negative beta-lactamases was examined. The dual-action cephalosporins tested were the ester Ro 23-9424; the carbamates Ro 25-2016, Ro 25-4095, and Ro 25-4835; and the tertiary amine Ro 25-0534. Also tested were cephalosporins with similar side chains (cefotaxime, desacetylcefotaxime, cephalothin, cephacetrile, and Ro 09-1227 [SR 0124]) and standard beta-lactams (penicillin G, cephaloridine). The beta-lactamases used were the plasmid-mediated TEM-1 and TEM-3 enzymes and the chromosomal AmpC. The cephacetrile-related compounds Ro 25-4095 and Ro 25-4835 were hydrolyzed by all three beta-lactamases with catalytic efficiencies (relative to penicillin G) ranging from approximately 5 (TEM-1, AmpC) to approximately 25 (TEM-3). The cephalothin-related Ro 25-2016 was also hydrolyzed by all three beta-lactamases, particularly the AmpC enzyme (relative catalytic efficiency, 110). The cefotaxime-related compounds Ro 25-0534 and Ro 23-9424 were hydrolyzed to any significant extent only by the TEM-3 enzyme (relative catalytic efficiencies, 1.2 and 4.7, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht H. A., Beskid G., Chan K. K., Christenson J. G., Cleeland R., Deitcher K. H., Georgopapadakou N. H., Keith D. D., Pruess D. L., Sepinwall J. Cephalosporin 3'-quinolone esters with a dual mode of action. J Med Chem. 1990 Jan;33(1):77–86. doi: 10.1021/jm00163a013. [DOI] [PubMed] [Google Scholar]
- Albrecht H. A., Beskid G., Christenson J. G., Durkin J. W., Fallat V., Georgopapadakou N. H., Keith D. D., Konzelmann F. M., Lipschitz E. R., McGarry D. H. Dual-action cephalosporins: cephalosporin 3'-quaternary ammonium quinolones. J Med Chem. 1991 Feb;34(2):669–675. doi: 10.1021/jm00106a031. [DOI] [PubMed] [Google Scholar]
- Albrecht H. A., Beskid G., Christenson J. G., Georgopapadakou N. H., Keith D. D., Konzelmann F. M., Pruess D. L., Rossman P. L., Wei C. C. Dual-action cephalosporins: cephalosporin 3'-quinolone carbamates. J Med Chem. 1991 Sep;34(9):2857–2864. doi: 10.1021/jm00113a026. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth T. P., Jr, White R. E., Tietjen R. A., Storrin R. J., Skuster J. R., Andersen J. A., McOsker C. C., Freedman R., Rourke F. J. Synthesis and antibacterial activity of new C-10 quinolonyl-cephem esters. J Antibiot (Tokyo) 1991 Feb;44(2):200–209. doi: 10.7164/antibiotics.44.200. [DOI] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Mechanism of inhibition of RTEM-2 beta-lactamase by cephamycins: relative importance of the 7 alpha-methoxy group and the 3' leaving group. Biochemistry. 1986 May 20;25(10):2934–2941. doi: 10.1021/bi00358a030. [DOI] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Bertasso A. Effects of quinolones on nucleoid segregation in Escherichia coli. Antimicrob Agents Chemother. 1991 Dec;35(12):2645–2648. doi: 10.1128/aac.35.12.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Bertasso A. Mechanisms of action of cephalosporin 3'-quinolone esters, carbamates, and tertiary amines in Escherichia coli. Antimicrob Agents Chemother. 1993 Mar;37(3):559–565. doi: 10.1128/aac.37.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Iyobe S., Inoue M., Mitsuhashi S. Purification and properties of a new beta-lactamase from Pseudomonas cepacia. Antimicrob Agents Chemother. 1980 Mar;17(3):355–358. doi: 10.1128/aac.17.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., O'Dea M. H., Gellert M. Cloning and simplified purification of Escherichia coli DNA gyrase A and B proteins. J Biol Chem. 1984 Jul 25;259(14):9199–9201. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nikaido H., Liu W., Rosenberg E. Y. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990 Feb;34(2):337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Pace J., Bertasso A., Georgopapadakou N. H. Escherichia coli resistant to cephalosporins and quinolones is still susceptible to the cephalosporin-quinolone ester Ro 23-9424. Antimicrob Agents Chemother. 1991 May;35(5):910–915. doi: 10.1128/aac.35.5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Zafaralla G., Manavathu E. K., Lerner S. A., Mobashery S. Elucidation of the role of arginine-244 in the turnover processes of class A beta-lactamases. Biochemistry. 1992 Apr 21;31(15):3847–3852. doi: 10.1021/bi00130a016. [DOI] [PubMed] [Google Scholar]


