Abstract
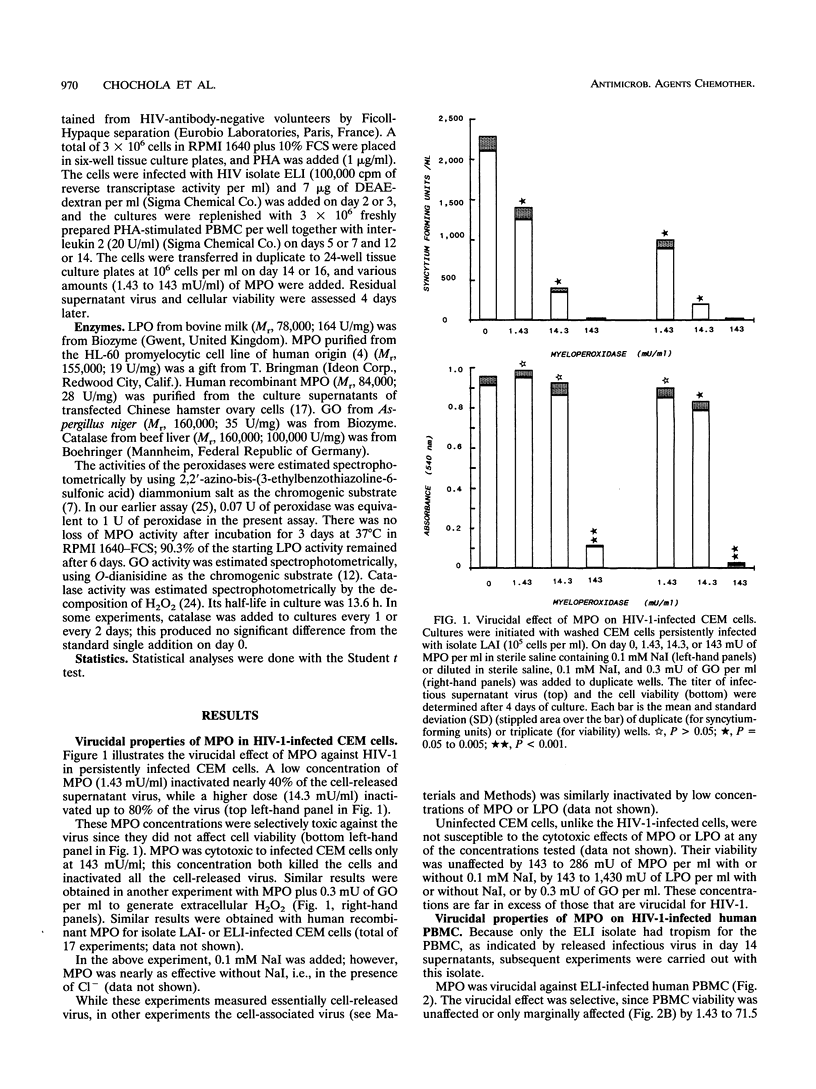
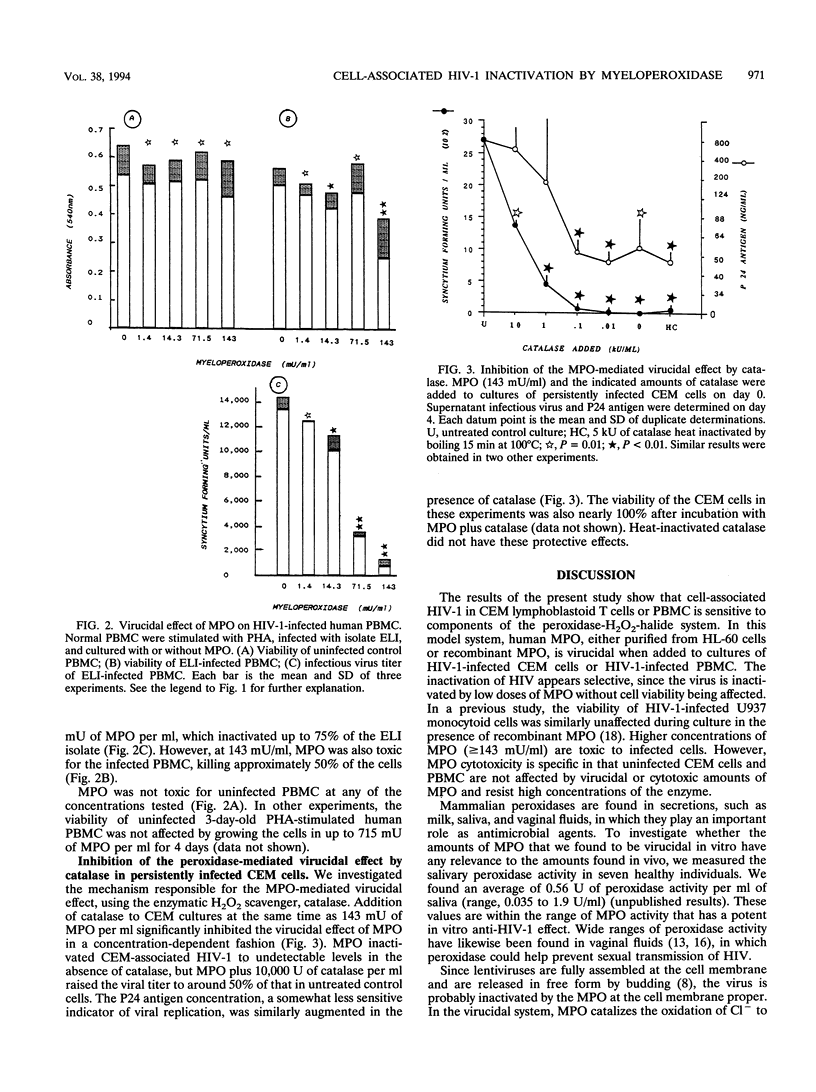
Myeloperoxidase is virucidal to human immunodeficiency virus type 1 (HIV-1) in the persistently infected CEM human T-cell line or in acutely infected human peripheral blood mononuclear cells, as judged by viral infectivity and P24 radioimmunoassay. HIV-1 was specifically inactivated by low doses of the human myeloperoxidase (1.4 to 14.3 mU/ml) and the cells were spared. A higher enzyme concentration (143 mU/m) was cytotoxic, but uninfected CEM cells and normal lymphocytes were resistant to > or = 143 mU of myeloperoxidase per ml. The enzyme was virucidal with the Cl- present in medium and did not require exogenous H2O2. Catalase, an antioxidant enzyme, partially inhibited the virucidal effect of myeloperoxidase. Hence, the H2O2 probably came from the HIV-infected cells themselves. These in vitro findings indicate that the myeloperoxidase system is capable of inactivating HIV-1 of infected cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589–601. [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Wells F. B., Mastrangeli A., Saltini C., Cantin A. M., Crystal R. G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989 Dec 2;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Casentini-Borocz D., Bringman T. Enzyme immunoconjugates utilizing glucose oxidase and myeloperoxidase are cytotoxic to Candida tropicalis. Antimicrob Agents Chemother. 1990 May;34(5):875–880. doi: 10.1128/aac.34.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M. J., Klebanoff S. J. Viricidal effect of stimulated human mononuclear phagocytes on human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5582–5585. doi: 10.1073/pnas.89.12.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Dionysius D. A., Grieve P. A., Vos A. C. Studies on the lactoperoxidase system: reaction kinetics and antibacterial activity using two methods for hydrogen peroxide generation. J Appl Bacteriol. 1992 Feb;72(2):146–153. doi: 10.1111/j.1365-2672.1992.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Cross C. E. Reactive oxygen species, antioxidants, and acquired immunodeficiency syndrome. Sense or speculation? Arch Intern Med. 1991 Jan;151(1):29–31. [PubMed] [Google Scholar]
- Heck D. E., Bisaccia E., Armus S., Laskin J. D. Production of hydrogen peroxide by cutaneous T-cell lymphoma following photopheresis with psoralens and ultraviolet light. Cancer Chemother Pharmacol. 1991;28(5):344–350. doi: 10.1007/BF00685687. [DOI] [PubMed] [Google Scholar]
- Ito H. O., Morizet J., Coulombel L., Goavec M., Rousseau V., Bernard A., Stanislawski M. An immunotoxin system intended for bone marrow purging composed of glucose oxidase and lactoperoxidase coupled to monoclonal antibody 097. Bone Marrow Transplant. 1989 Sep;4(5):519–527. [PubMed] [Google Scholar]
- Klebanoff S. J., Coombs R. W. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991 Jul 1;174(1):289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Coombs R. W. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. J Clin Invest. 1992 Jun;89(6):2014–2017. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J., Hillier S. L., Eschenbach D. A., Waltersdorph A. M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991 Jul;164(1):94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- Moguilevsky N., Garcia-Quintana L., Jacquet A., Tournay C., Fabry L., Piérard L., Bollen A. Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem. 1991 May 8;197(3):605–614. doi: 10.1111/j.1432-1033.1991.tb15950.x. [DOI] [PubMed] [Google Scholar]
- Moguilevsky N., Steens M., Thiriart C., Prieels J. P., Thiry L., Bollen A. Lethal oxidative damage to human immunodeficiency virus by human recombinant myeloperoxidase. FEBS Lett. 1992 May 18;302(3):209–212. doi: 10.1016/0014-5793(92)80442-j. [DOI] [PubMed] [Google Scholar]
- Pourtois M., Binet C., Van Tieghem N., Courtois P. R., Vandenabbeele A., Thirty L. Saliva can contribute in quick inhibition of HIV infectivity. AIDS. 1991 May;5(5):598–600. [PubMed] [Google Scholar]
- Rocancourt D., Bonnerot C., Jouin H., Emerman M., Nicolas J. F. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990 Jun;64(6):2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988 Dec;56(12):3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski T. P., Nathan C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991 Feb 1;51(3):794–798. [PubMed] [Google Scholar]
- Yamaguchi Y., Semmel M., Stanislawski L., Strosberg A. D., Stanislawski M. Virucidal effects of glucose oxidase and peroxidase or their protein conjugates on human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993 Jan;37(1):26–31. doi: 10.1128/aac.37.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]


