Abstract
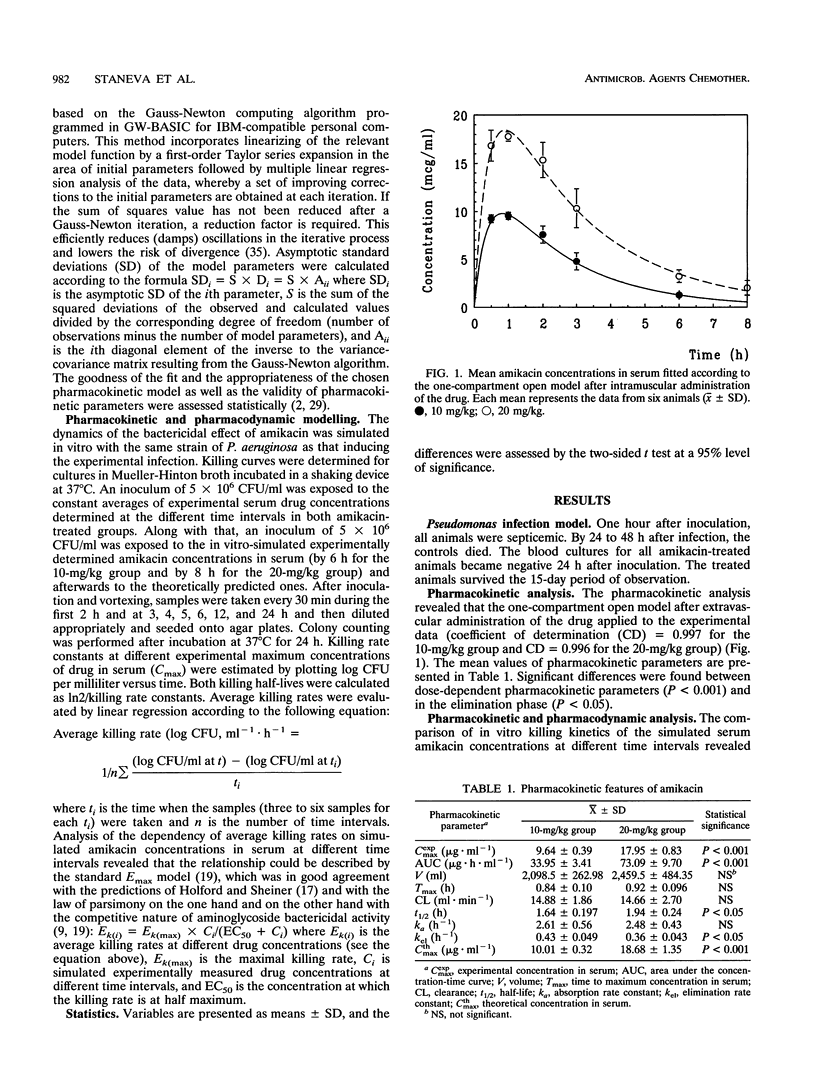
The efficiencies of two dosage schedules of amikacin (2 x 10 mg/kg of body weight per 24 h and 1 x 20 mg/kg/24 h intramuscularly for 5 days) against Pseudomonas aeruginosa sepsis in rabbits were compared. Blood samples were drawn at various times after the first application, and amikacin concentrations in serum were assayed microbiologically. The dynamics of the bactericidal effect of amikacin was simulated in vitro with the same strain of P. aeruginosa. No regrowth was found with the 20-mg/kg dose when the bacterial inoculum was in contact with experimental and theoretically predicted serum amikacin concentrations. The killing effect was present even when the drug levels decreased considerably below the MIC. The interrelationship between simulated amikacin concentrations in serum and the corresponding average killing rates was described appropriately by the standard Emax model. The higher amikacin dose performed its bacterial effect faster and the drug persisted longer in the blood. The two amikacin regimens were therapeutically equivalent, but the once-daily schedule had some advantages over the twice-daily drug administration which became evident when both the pharmacokinetic and the pharmacodynamic parameters of the drug were considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Bryan L. E. General mechanisms of resistance to antibiotics. J Antimicrob Chemother. 1988 Jul;22 (Suppl A):1–15. doi: 10.1093/jac/22.supplement_a.1. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Vogelman B. The postantibiotic effect. Ann Intern Med. 1987 Jun;106(6):900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Jackson G. G., Lolans V. T., Livermore D. M. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990 Aug;162(2):414–420. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother. 1991 Jan;35(1):117–123. doi: 10.1128/aac.35.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHertogh D. A., Lerner S. A. Correlation of aminoglycoside resistance with the KmS and Vmax/Km ratios of enzymatic modification of aminoglycosides by 2''-O-nucleotidyltransferase. Antimicrob Agents Chemother. 1985 Apr;27(4):670–671. doi: 10.1128/aac.27.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimodt-Møller N. Correlation of in vitro activity and pharmacokinetic parameters with effect in vivo for antibiotics. Observations from experimental pneumococcus infection. Dan Med Bull. 1988 Oct;35(5):422–437. [PubMed] [Google Scholar]
- Garraffo R., Dellamonica P., Drugeon H. B., Etesse H., Lapalus P. A new approach to optimal antibiotic dosage regimen by coupling pharmacokinetics and killing curve parameters. Methods Find Exp Clin Pharmacol. 1990 Jun;12(5):325–332. [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A., Brugger H. P., Feller C., Vastola A. P., Brandel J. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis. 1983 May;147(5):910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Feller-Segessenmann C. In-vivo assessment of in-vitro killing patterns of Pseudomonas aeruginosa. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):201–206. doi: 10.1093/jac/15.suppl_a.201. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Vastola A. P., Brandel J., Craig W. A. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis. 1982 Nov;146(5):691–697. doi: 10.1093/infdis/146.5.691. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Bellido F. Antibiotic uptake: unusual results for unusual molecules. J Antimicrob Chemother. 1992 Mar;29(3):235–239. doi: 10.1093/jac/29.3.235. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Kinetics of pharmacologic response. Pharmacol Ther. 1982;16(2):143–166. doi: 10.1016/0163-7258(82)90051-1. [DOI] [PubMed] [Google Scholar]
- Jackson G. G., Lolans V. T., Daikos G. L. The inductive role of ionic binding in the bactericidal and postexposure effects of aminoglycoside antibiotics with implications for dosing. J Infect Dis. 1990 Aug;162(2):408–413. doi: 10.1093/infdis/162.2.408. [DOI] [PubMed] [Google Scholar]
- Jackson S. H., Jamieson M. J., Johnston A., Shepherd A. M. The analysis of dose-response curves--a practical approach. Br J Clin Pharmacol. 1987 Feb;23(2):199–205. doi: 10.1111/j.1365-2125.1987.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusnik J. E., Hackbarth C. J., Chambers H. F., Carpenter T., Sande M. A. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis. 1988 Jul;158(1):7–12. doi: 10.1093/infdis/158.1.7. [DOI] [PubMed] [Google Scholar]
- Kovarik J. M., Hoepelman I. M., Verhoef J. Once-daily aminoglycoside administration: new strategies for an old drug. Eur J Clin Microbiol Infect Dis. 1989 Sep;8(9):761–769. doi: 10.1007/BF02185842. [DOI] [PubMed] [Google Scholar]
- MacArthur R. D., Lolans V., Zar F. A., Jackson G. G. Biphasic, concentration-dependent and rate-limited, concentration-independent bacterial killing by an aminoglycoside antibiotic. J Infect Dis. 1984 Nov;150(5):778–779. doi: 10.1093/infdis/150.5.778. [DOI] [PubMed] [Google Scholar]
- Mattie H., Craig W. A., Pechère J. C. Determinants of efficacy and toxicity of aminoglycosides. J Antimicrob Chemother. 1989 Sep;24(3):281–293. doi: 10.1093/jac/24.3.281. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Nordström L., Ringberg H., Cronberg S., Tjernström O., Walder M. Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity? J Antimicrob Chemother. 1990 Jan;25(1):159–173. doi: 10.1093/jac/25.1.159. [DOI] [PubMed] [Google Scholar]
- Parker S. E., Davey P. G. Practicalities of once-daily aminoglycoside dosing. J Antimicrob Chemother. 1993 Jan;31(1):4–8. doi: 10.1093/jac/31.1.4. [DOI] [PubMed] [Google Scholar]
- Powell S. H., Thompson W. L., Luthe M. A., Stern R. C., Grossniklaus D. A., Bloxham D. D., Groden D. L., Jacobs M. R., DiScenna A. O., Cash H. A. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983 May;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Swanson D. J., Smith I. L. Dual individualization: antibiotic dosage calculation from the integration of in-vitro pharmacodynamics and in-vivo pharmacokinetics. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):47–57. doi: 10.1093/jac/15.suppl_a.47. [DOI] [PubMed] [Google Scholar]
- Terziivanov D., Gerova Z., Vlahov V., Merdzhanov A., Damjanov D. Pharmacokinetics and quantitative characterization of cefotiam excretion after intravenous administration to patients after cholecystectomy. Eur J Clin Pharmacol. 1986;30(4):439–444. doi: 10.1007/BF00607957. [DOI] [PubMed] [Google Scholar]
- Verpooten G. A., Giuliano R. A., Verbist L., Eestermans G., De Broe M. E. Once-daily dosing decreases renal accumulation of gentamicin and netilmicin. Clin Pharmacol Ther. 1989 Jan;45(1):22–27. doi: 10.1038/clpt.1989.4. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Craig W. A. Kinetics of antimicrobial activity. J Pediatr. 1986 May;108(5 Pt 2):835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- Wood C. A., Norton D. R., Kohlhepp S. J., Kohnen P. W., Porter G. A., Houghton D. C., Brummett R. E., Bennett W. M., Gilbert D. N. The influence of tobramycin dosage regimens on nephrotoxicity, ototoxicity, and antibacterial efficacy in a rat model of subcutaneous abscess. J Infect Dis. 1988 Jul;158(1):13–22. doi: 10.1093/infdis/158.1.13. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Nishikawa S., Sugiyama T., Kobayashi T., Shimada H., Tomonaga F., Ohdo S., Ogawa N., Nakano S. Influence of circadian-stage-dependent dosing schedule on nephrotoxicity and pharmacokinetics of isepamicin in rats. Antimicrob Agents Chemother. 1993 Sep;37(9):2042–2043. doi: 10.1128/aac.37.9.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]


