Abstract
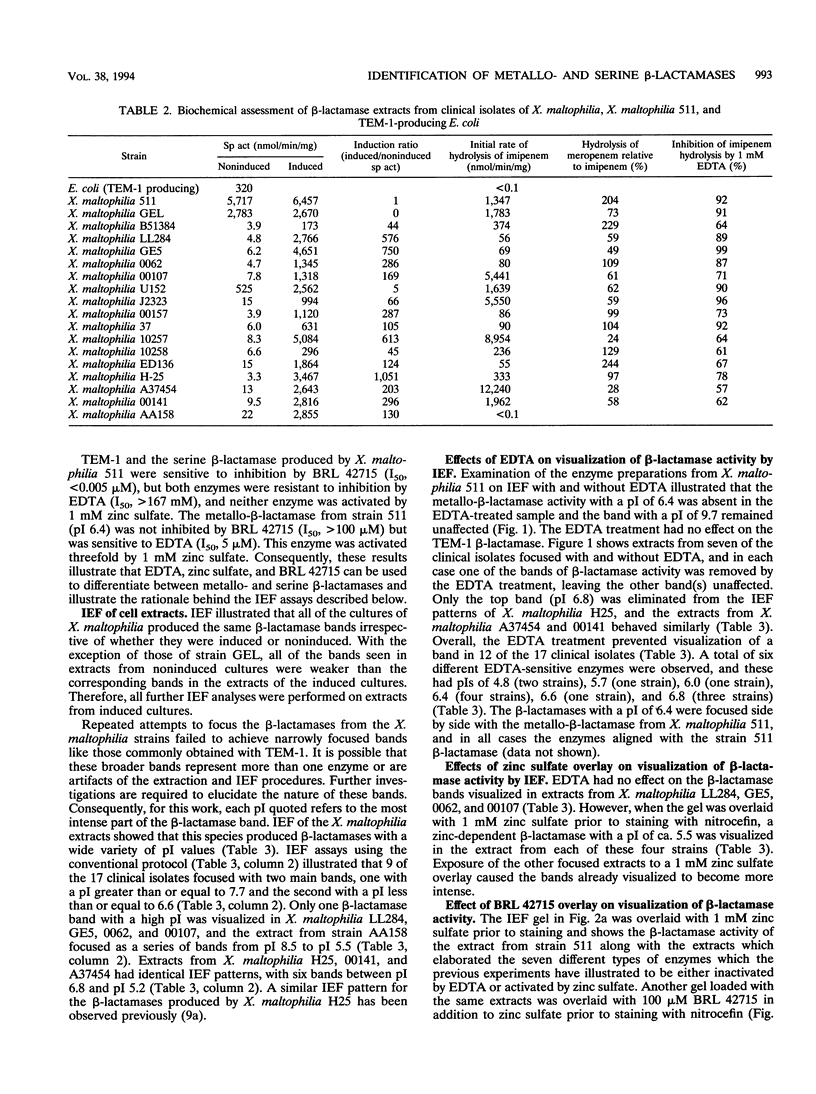
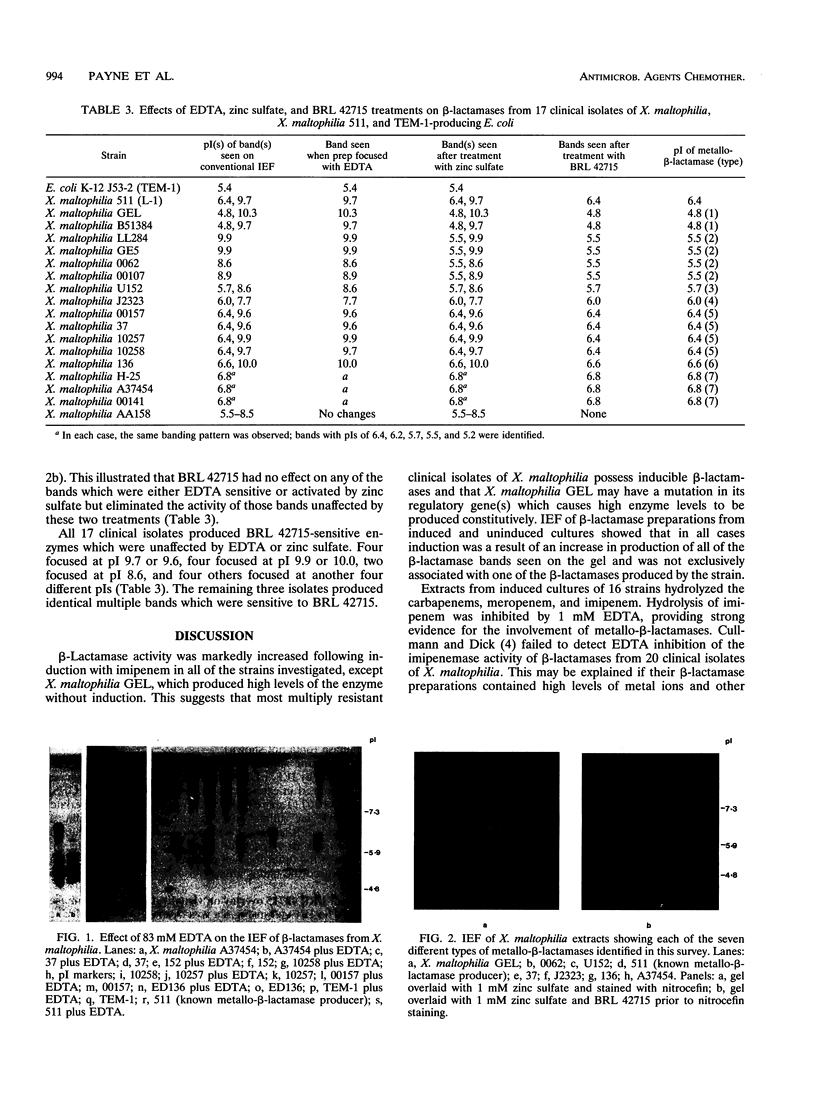
Simple methods to detect, identify, and differentiate metallo- and serine beta-lactamases were developed and used to differentiate enzymes produced by 17 clinical isolates of Xanthomonas maltophilia. All isolates exhibited beta-lactamase activity, and in 16 strains this was induced by imipenem. All but one isolate hydrolyzed imipenem (and meropenem), and in all cases this activity was inhibited by 1 mM EDTA. The metallo- and serine beta-lactamases in the cell extracts were distinguished on isoelectric focusing (IEF) gels by using the following procedures. (i) Cell lysates were preincubated with 83 mM EDTA prior to IEF and subsequent visualization with nitrocefin, and (ii) after IEF, the gels were overlaid with either 1 mM zinc sulfate or 100 microM BRL 42715 before staining with nitrocefin. Bands of beta-lactamase activity which were removed by BRL 42715 but unaffected by EDTA or zinc sulfate were categorized as serine beta-lactamases. Bands which were unaffected by BRL 42715 but inhibited by EDTA or enhanced by zinc sulfate were classified as metallo-beta-lactamases. By using this approach, seven metallo-beta-lactamases were differentiated with pI values of 4.8 (two strains), 5.5 (four strains), 5.7 (one strain), 6.0 (one strain), 6.4 (four strains), 6.6 (one strain), and 6.8 (three strains). The metallo-beta-lactamase band with a pI of 6.4 aligned with the recently characterized metallo-beta-lactamase from X. maltophilia 511. Heterogeneity was also observed for the serine beta-lactamases: 14 isolates elaborated serine beta-lactamase activity which focused with major bands with at least eight different pIs. The remaining three strains produced serine beta-lactamases which focused with five distinct bands with pIs of 6.4, 6.2, 5.7, 5.5, and 5.2. We conclude that X. maltophilia produces many types of metallo- and serine beta-lactamases distinguishable by these new methods and that the previously reported L-1 and L-2 enzymes are not solely representative of the beta-lactamases produced by this species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akova M., Bonfiglio G., Livermore D. M. Susceptibility to beta-lactam antibiotics of mutant strains of Xanthomonas maltophilia with high- and low-level constitutive expression of L1 and L2 beta-lactamases. J Med Microbiol. 1991 Oct;35(4):208–213. doi: 10.1099/00222615-35-4-208. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Emanuel E. L., Gagnon J., Waley S. G. The production and molecular properties of the zinc beta-lactamase of Pseudomonas maltophilia IID 1275. Biochem J. 1985 Aug 1;229(3):791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K., Griffin D. R., Page J. W., Upshon P. A. In vitro evaluation of BRL 42715, a novel beta-lactamase inhibitor. Antimicrob Agents Chemother. 1989 Sep;33(9):1580–1587. doi: 10.1128/aac.33.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Dick W. Heterogeneity of beta-lactamase production in Pseudomonas maltophilia, a nosocomial pathogen. Chemotherapy. 1990;36(2):117–126. doi: 10.1159/000238757. [DOI] [PubMed] [Google Scholar]
- Elting L. S., Khardori N., Bodey G. P., Fainstein V. Nosocomial infection caused by Xanthomonas maltophilia: a case-control study of predisposing factors. Infect Control Hosp Epidemiol. 1990 Mar;11(3):134–138. doi: 10.1086/646136. [DOI] [PubMed] [Google Scholar]
- Felici A., Amicosante G., Oratore A., Strom R., Ledent P., Joris B., Fanuel L., Frère J. M. An overview of the kinetic parameters of class B beta-lactamases. Biochem J. 1993 Apr 1;291(Pt 1):151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Payne D. J., Coleman K., Cramp R. The automated in-vitro assessment of beta-lactamase inhibitors. J Antimicrob Chemother. 1991 Nov;28(5):775–776. doi: 10.1093/jac/28.5.775. [DOI] [PubMed] [Google Scholar]
- Payne D. J. Metallo-beta-lactamases--a new therapeutic challenge. J Med Microbiol. 1993 Aug;39(2):93–99. doi: 10.1099/00222615-39-2-93. [DOI] [PubMed] [Google Scholar]
- Payne D. J., Woodford N., Amyes S. G. Characterization of the plasmid mediated beta-lactamase BIL-1. J Antimicrob Chemother. 1992 Aug;30(2):119–127. doi: 10.1093/jac/30.2.119. [DOI] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]