Abstract
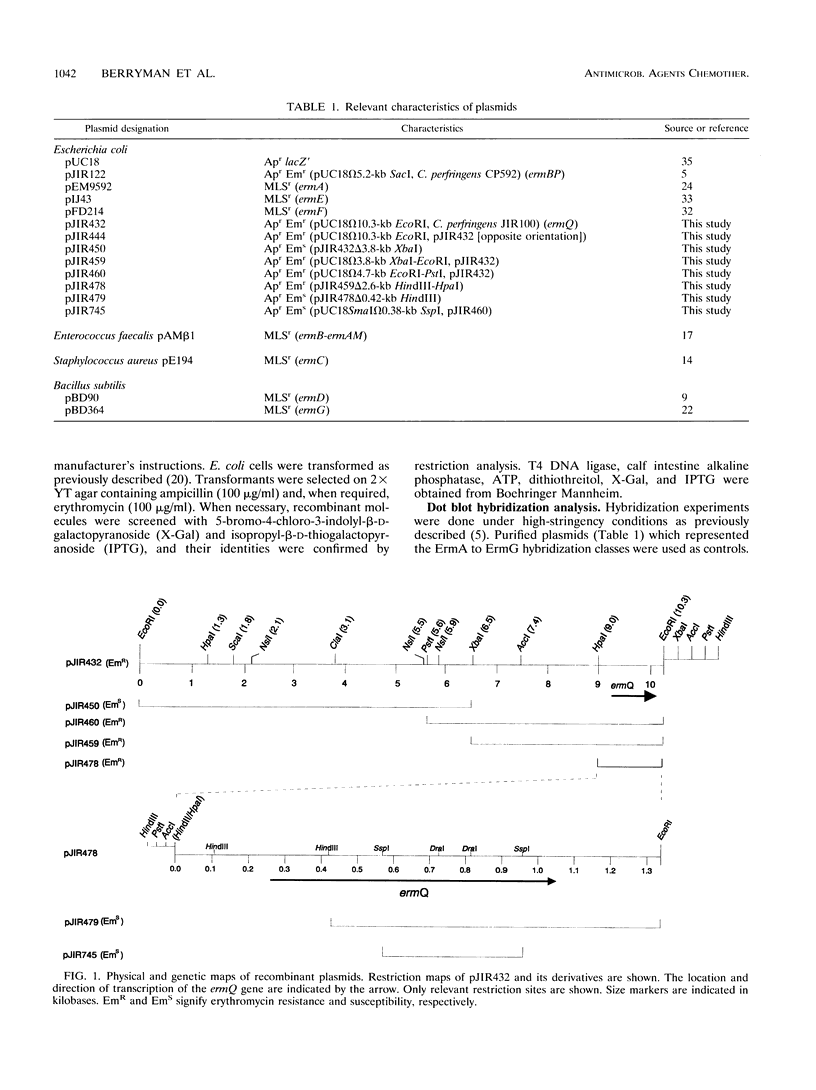
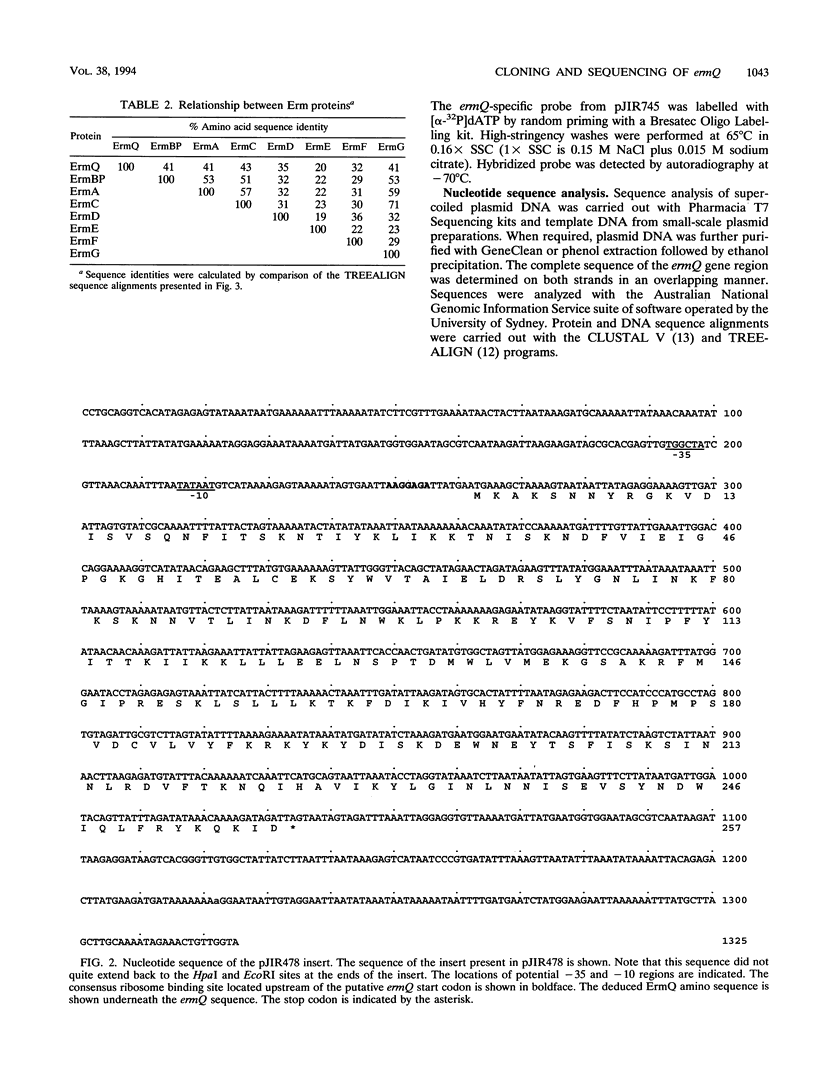
The erythromycin resistance determinant from Clostridium perfringens JIR100 has been cloned, sequenced, and shown to be expressed in Escherichia coli. An open reading frame with sequence similarity to erm genes from other bacteria was identified and designated the ermQ gene. On the basis of comparative sequence analysis, it was concluded that the ermQ gene represented a new Erm hybridization class, designated ErmQ. Genes belonging to the ErmQ class were found to be widespread in C. perfringens, since 30 of 38 macrolide-lincosamide-streptogramin B-resistant C. perfringens strains, from diverse sources, hybridized to an ermQ-specific gene probe. The ermQ gene therefore represents the most common erythromycin resistance determinant in C. perfringens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Berryman D. I., Rood J. I. Hybridization analysis of the class P tetracycline resistance determinant from the Clostridium perfringens R-plasmid, pCW3. Plasmid. 1988 Mar;19(2):113–120. doi: 10.1016/0147-619x(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Abraham L. J., Rood J. I. Cloning and analysis of the Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid. 1985 May;13(3):155–162. doi: 10.1016/0147-619x(85)90038-1. [DOI] [PubMed] [Google Scholar]
- Abraham L. J., Rood J. I. Molecular analysis of transferable tetracycline resistance plasmids from Clostridium perfringens. J Bacteriol. 1985 Feb;161(2):636–640. doi: 10.1128/jb.161.2.636-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannam T. L., Rood J. I. Relationship between the Clostridium perfringens catQ gene product and chloramphenicol acetyltransferases from other bacteria. Antimicrob Agents Chemother. 1991 Mar;35(3):471–476. doi: 10.1128/aac.35.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman D. I., Rood J. I. Cloning and hybridization analysis of ermP, a macrolide-lincosamide-streptogramin B resistance determinant from Clostridium perfringens. Antimicrob Agents Chemother. 1989 Aug;33(8):1346–1353. doi: 10.1128/aac.33.8.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefort G., Magot M., Ionesco H., Sebald M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid. 1977 Nov;1(1):52–66. doi: 10.1016/0147-619x(77)90008-7. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986 Nov;205(2):291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- Docherty A., Grandi G., Grandi R., Gryczan T. J., Shivakumar A. G., Dubnau D. Naturally occurring macrolide-lincosamide-streptogramin B resistance in Bacillus licheniformis. J Bacteriol. 1981 Jan;145(1):129–137. doi: 10.1128/jb.145.1.129-137.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A. S., Bannam T. L., Rood J. I. Comparative sequence analysis of the catB gene from Clostridium butyricum. Antimicrob Agents Chemother. 1992 Nov;36(11):2548–2551. doi: 10.1128/aac.36.11.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Berger-Bächi B., Kayser F. H. Genetic characterization of a Clostridium difficile erythromycin-clindamycin resistance determinant that is transferable to Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Jul;31(7):1039–1045. doi: 10.1128/aac.31.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Francis B. S. Taxonomy of the Clostridia: ribosomal ribonucleic acid homologies among the species. J Gen Microbiol. 1975 Jun;88(2):229–244. doi: 10.1099/00221287-88-2-229. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Physical and genetic analyses of streptococcal plasmid pAM beta 1 and cloning of its replication region. J Bacteriol. 1984 Feb;157(2):445–453. doi: 10.1128/jb.157.2.445-453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991 Jul;35(7):1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991 Jul;35(7):1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M., Mohan S., Dubnau D. Cloning and analysis of ermG, a new macrolide-lincosamide-streptogramin B resistance element from Bacillus sphaericus. J Bacteriol. 1987 Jan;169(1):340–350. doi: 10.1128/jb.169.1.340-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Löfdahl S. Transposition of Tn554 does not generate a target duplication. Nature. 1984 Jan 19;307(5948):292–294. doi: 10.1038/307292a0. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yamakawa K., Nakashio S., Kamiya S., Nishida S. Correlation between susceptibility to chloramphenicol, tetracycline and clindamycin, and serogroups of Clostridium difficile. Med Microbiol Immunol. 1987;176(2):79–82. doi: 10.1007/BF00200678. [DOI] [PubMed] [Google Scholar]
- Rood J. I., Buddle J. R., Wales A. J., Sidhu R. The occurrence of antibiotic resistance in Clostridium perfringens from pigs. Aust Vet J. 1985 Aug;62(8):276–279. doi: 10.1111/j.1751-0813.1985.tb14251.x. [DOI] [PubMed] [Google Scholar]
- Rood J. I., Cole S. T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991 Dec;55(4):621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Jefferson S., Bannam T. L., Wilkie J. M., Mullany P., Wren B. W. Hybridization analysis of three chloramphenicol resistance determinants from Clostridium perfringens and Clostridium difficile. Antimicrob Agents Chemother. 1989 Sep;33(9):1569–1574. doi: 10.1128/aac.33.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Maher E. A., Somers E. B., Campos E., Duncan C. L. Isolation and characterization of multiply antibiotic-resistant Clostridum perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978 May;13(5):871–880. doi: 10.1128/aac.13.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I. Transferable tetracycline resistance in Clostridium perfringens strains of porcine origin. Can J Microbiol. 1983 Oct;29(10):1241–1246. doi: 10.1139/m83-193. [DOI] [PubMed] [Google Scholar]
- Smith C. J. Development and use of cloning systems for Bacteroides fragilis: cloning of a plasmid-encoded clindamycin resistance determinant. J Bacteriol. 1985 Oct;164(1):294–301. doi: 10.1128/jb.164.1.294-301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Comparative in vitro bactericidal activity of 24 antimicrobial drugs against Clostridium perfringens. Chemotherapy. 1990;36(2):127–135. doi: 10.1159/000238758. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jaworowski A., Poulis M. I. Amplification of the respiratory NADH dehydrogenase of Escherichia coli by gene cloning. Gene. 1978 Sep;4(1):25–36. doi: 10.1016/0378-1119(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Youngman P. J., Perkins J. B., Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]


