Abstract
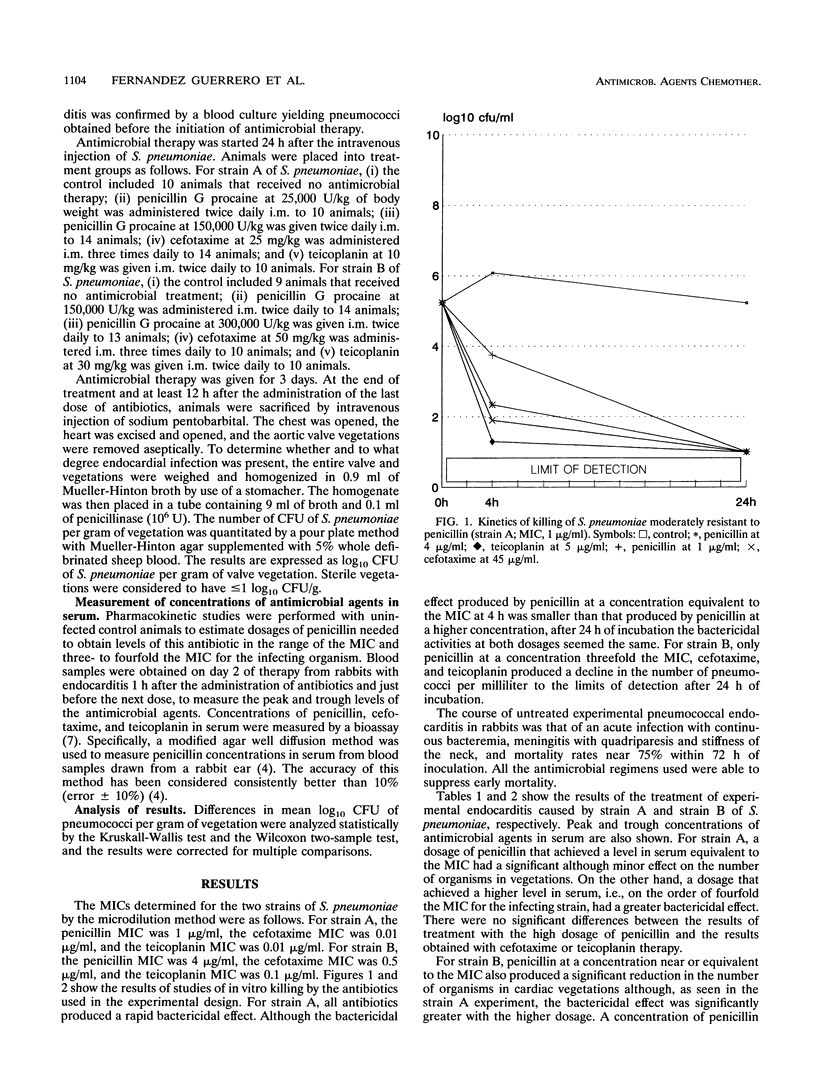
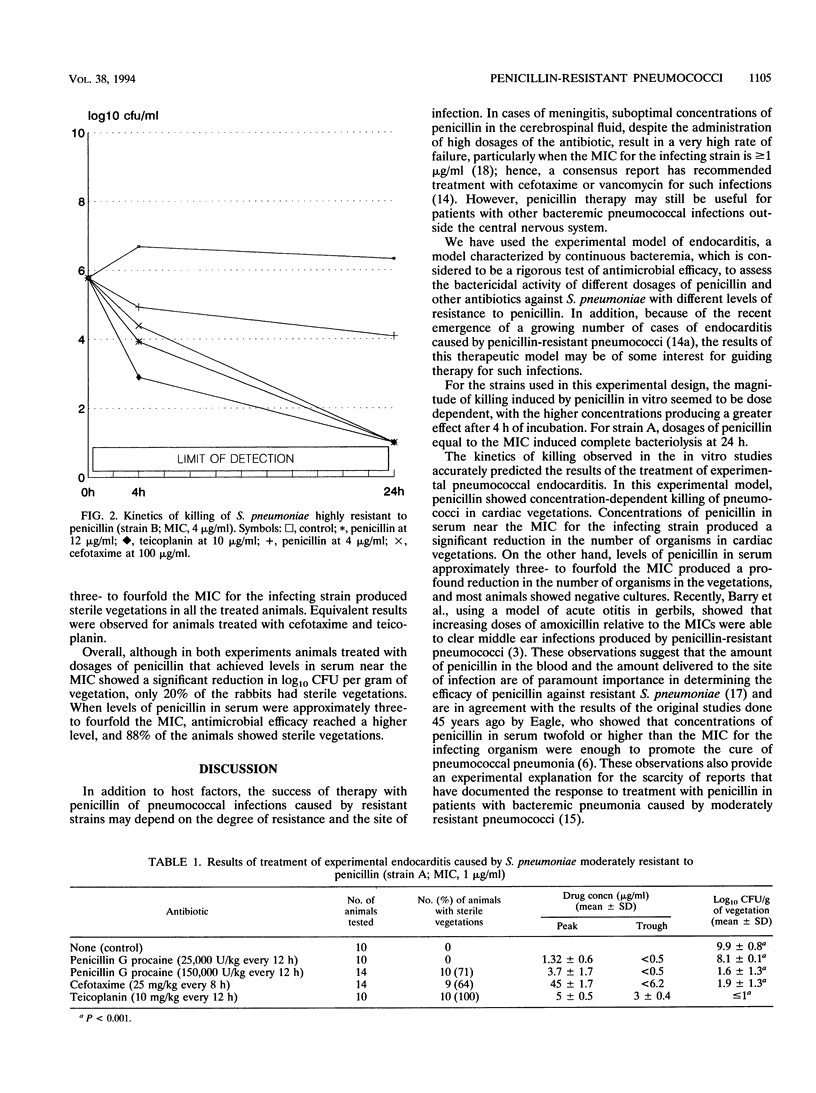
Using two strains of pneumococci for which MICs of penicillin were 1 and 4 micrograms/ml, those of cefotaxime were 0.01 and 0.5 micrograms/ml, and those of teicoplanin were 0.01 and 0.1 micrograms/ml, we studied the efficacy of different dosages of penicillin, cefotaxime, and teicoplanin in the treatment of experimental pneumococcal endocarditis in rabbits. Animals treated with dosages of penicillin G procaine needed to achieve levels in serum near the MIC for pneumococci showed a significant reduction in log10 CFU per gram of vegetation, as compared with the control (P < 0.001), although only 20% of the animals showed sterile vegetations. When levels of penicillin in serum were in the range of three- to fourfold the MIC, a greater reduction in log10 CFU per gram of vegetation was seen, and 88% of the animals showed sterile vegetations. Only the regimen of penicillin that provided concentrations in serum above the MIC throughout the interval between two doses provided constant sterilization of the cardiac vegetations. Dosages of cefotaxime and teicoplanin selected to achieve concentrations in serum equivalent to that obtained in humans during treatment resulted in levels of antimicrobial agents in serum hundreds or thousands of times higher than the MICs for the infecting strains. In terms of antimicrobial efficacy, cefotaxime and teicoplanin were equivalent to regimens with high dosages of penicillin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987 Aug;6(4):367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- Barry B., Muffat-Joly M., Gehanno P., Pocidalo J. J. Effect of increased dosages of amoxicillin in treatment of experimental middle ear otitis due to penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1993 Aug;37(8):1599–1603. doi: 10.1128/aac.37.8.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato R. F., Swenson J. M., McKinley G. A., Hochstein L., Wallman A. A., Cleri D. J., Mastellone A. J., Fredericks L., Gonzalez L., Pincus D. H. Quantitative antimicrobial susceptibility test for Streptococcus pneumoniae using inoculum supplemented with whole defibrinated sheep blood. J Clin Microbiol. 1987 Sep;25(9):1753–1756. doi: 10.1128/jcm.25.9.1753-1756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham D., Foxall P., O'Hare M., Grüneberg R. The bactericidal activity of vancomycin and teicoplanin against Streptococcus pneumoniae. Scand J Infect Dis Suppl. 1990;72:20–25. [PubMed] [Google Scholar]
- Garrison P. K., Freedman L. R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970 Jun;42(6):394–410. [PMC free article] [PubMed] [Google Scholar]
- Hess J., Dankert J., Durack D. Significance of penicillin tolerance in vivo: prevention of experimental Streptococcus sanguis endocarditis. J Antimicrob Chemother. 1983 Jun;11(6):555–564. doi: 10.1093/jac/11.6.555. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992 Jul;15(1):119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liñares J., Pallares R., Alonso T., Perez J. L., Ayats J., Gudiol F., Viladrich P. F., Martin R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979-1990). Clin Infect Dis. 1992 Jul;15(1):99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Nelson J. D., Kaplan S. L., Overturf G. D., Rodriguez W. J., Steele R. W. Consensus report: antimicrobial therapy for bacterial meningitis in infants and children. Pediatr Infect Dis J. 1987 Jun;6(6):501–505. [PubMed] [Google Scholar]
- Pallares R., Gudiol F., Liñares J., Ariza J., Rufi G., Murgui L., Dorca J., Viladrich P. F. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N Engl J Med. 1987 Jul 2;317(1):18–22. doi: 10.1056/NEJM198707023170104. [DOI] [PubMed] [Google Scholar]
- Pelletier L. L., Jr, Petersdorf R. G., Nielson K. Chemotherapy of experimental streptococcal endocarditis. V. Effect of duration of infection and retained intracardiac catheter on response to treatment. J Lab Clin Med. 1976 Apr;87(4):692–702. [PubMed] [Google Scholar]
- Soriano F. Optimal dosage of beta-lactam antibiotics: time above the MIC and inoculum effect. J Antimicrob Chemother. 1992 Nov;30(5):566–569. doi: 10.1093/jac/30.5.567. [DOI] [PubMed] [Google Scholar]
- Viladrich P. F., Gudiol F., Liñares J., Rufi G., Ariza J., Pallares R. Characteristics and antibiotic therapy of adult meningitis due to penicillin-resistant pneumococci. Am J Med. 1988 May;84(5):839–846. doi: 10.1016/0002-9343(88)90061-7. [DOI] [PubMed] [Google Scholar]


