Abstract
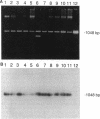
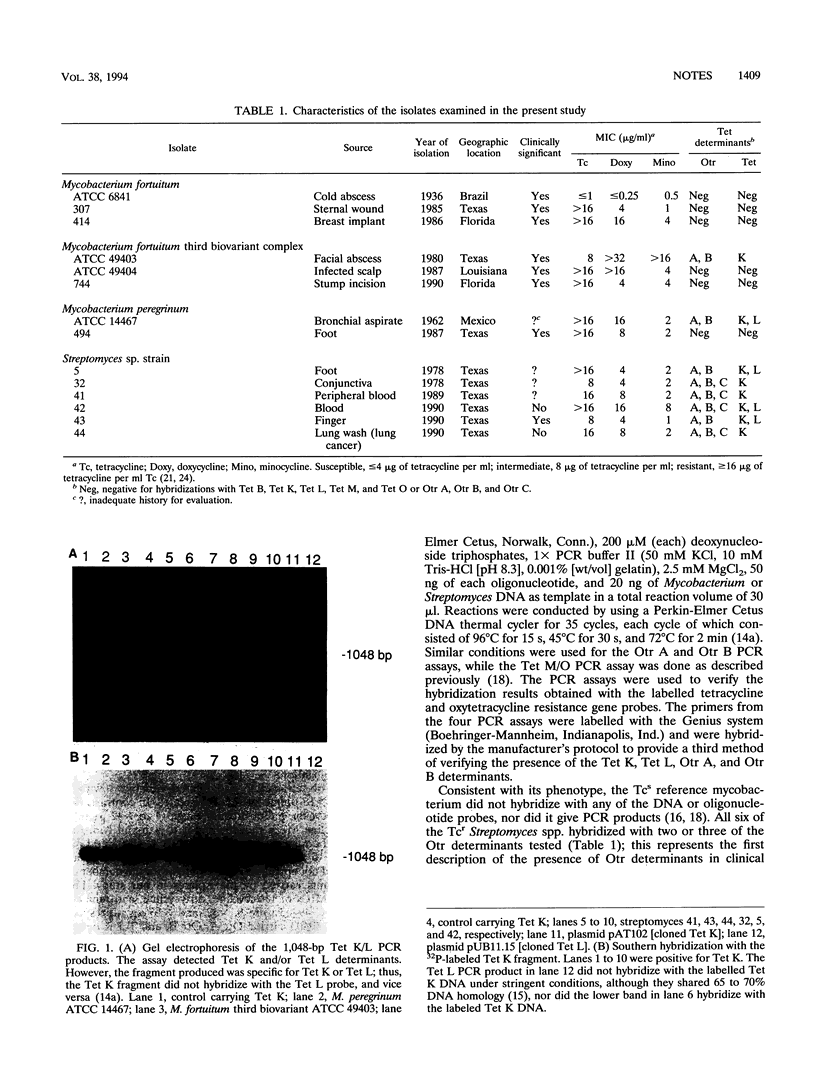
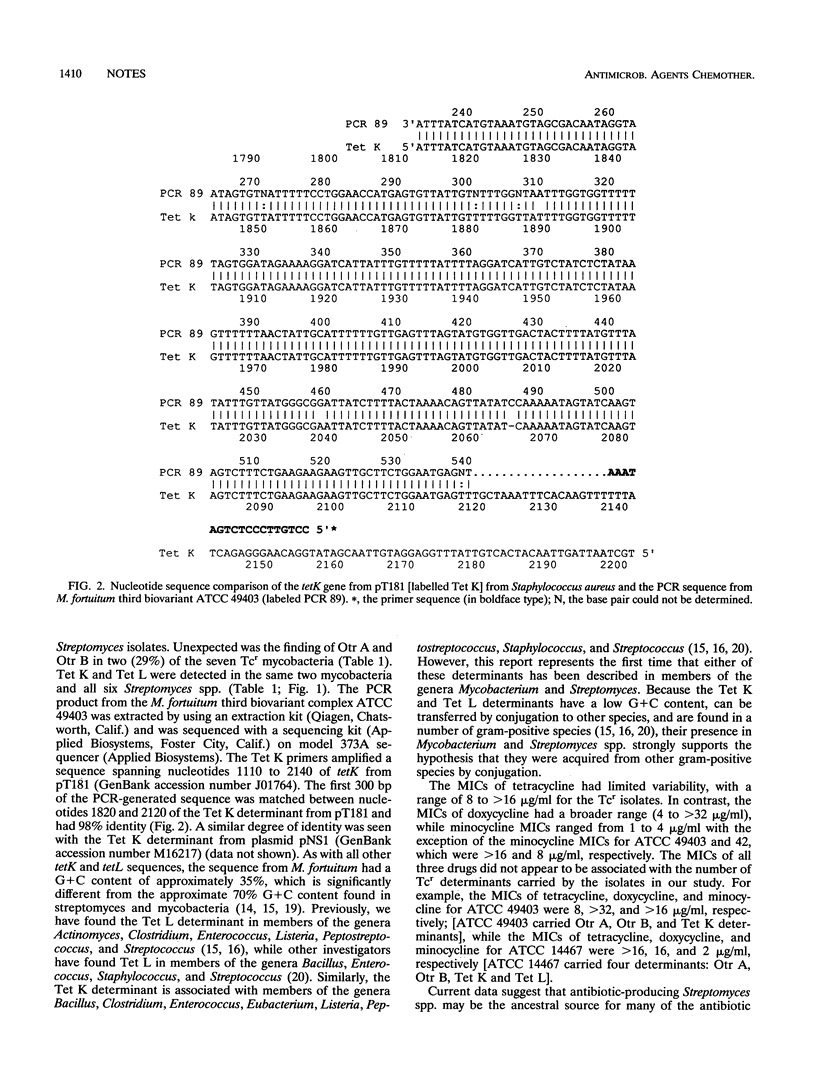
Two of seven tetracycline-resistant (Tcr) Mycobacterium fortuitum group isolates and six Tcr clinical Streptomyces isolates carried gram-positive Tcr determinants (Tet K and Tet L) and Streptomyces resistance determinants (Otr A, Otr B, and Otr C). This represents the first documentation of the acquisition by mycobacteria of determinants coding for antibiotic resistance and suggests the potential for the spread of antibiotic resistance determinants within mycobacterial species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler M. J., Friend E. J., Hunter I. S., Kaczmarek F. S., Sugden D. A., Warren M. Molecular cloning of resistance genes and architecture of a linked gene cluster involved in biosynthesis of oxytetracycline by Streptomyces rimosus. Mol Gen Genet. 1989 Jan;215(2):231–238. doi: 10.1007/BF00339722. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Tuberculosis: the return of an old enemy. Crit Rev Microbiol. 1993;19(1):1–16. doi: 10.3109/10408419309113520. [DOI] [PubMed] [Google Scholar]
- Dalovisio J. R., Pankey G. A., Wallace R. J., Jones D. B. Clinical usefulness of amikacin and doxycycline in the treatment of infection due to Mycobacterium fortuitum and Mycobacterium chelonei. Rev Infect Dis. 1981 Sep-Oct;3(5):1068–1074. doi: 10.1093/clinids/3.5.1068. [DOI] [PubMed] [Google Scholar]
- Dittrich W., Schrempf H. The unstable tetracycline resistance gene of Streptomyces lividans 1326 encodes a putative protein with similarities to translational elongation factors and Tet(M) and Tet(O) proteins. Antimicrob Agents Chemother. 1992 May;36(5):1119–1124. doi: 10.1128/aac.36.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D., McDowall K. J., Butler M. J., Hunter I. S. Characterization of an oxytetracycline-resistance gene, otrA, of Streptomyces rimosus. Mol Microbiol. 1991 Dec;5(12):2923–2933. doi: 10.1111/j.1365-2958.1991.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Edlin B. R., Tokars J. I., Grieco M. H., Crawford J. T., Williams J., Sordillo E. M., Ong K. R., Kilburn J. O., Dooley S. W., Castro K. G. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992 Jun 4;326(23):1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- Hellyer T. J., Brown I. N., Dale J. W., Easmon C. S. Plasmid analysis of Mycobacterium avium-intracellulare (MAI) isolated in the United Kingdom from patients with and without AIDS. J Med Microbiol. 1991 Apr;34(4):225–231. doi: 10.1099/00222615-34-4-225. [DOI] [PubMed] [Google Scholar]
- Hermans J., Martin C., Huijberts G. N., Goosen T., de Bont J. A. Transformation of Mycobacterium aurum and Mycobacterium smegmatis with the broad host-range gram-negative cosmid vector pJRD215. Mol Microbiol. 1991 Jun;5(6):1561–1566. doi: 10.1111/j.1365-2958.1991.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Heym B., Cole S. T. Isolation and characterization of isoniazid-resistant mutants of Mycobacterium smegmatis and M. aurum. Res Microbiol. 1992 Sep;143(7):721–730. doi: 10.1016/0923-2508(92)90067-x. [DOI] [PubMed] [Google Scholar]
- Hull S. I., Wallace R. J., Jr, Bobey D. G., Price K. E., Goodhines R. A., Swenson J. M., Silcox V. A. Presence of aminoglycoside acetyltransferase and plasmids in Mycobacterium fortuitum. Lack of correlation with intrinsic aminoglycoside resistance. Am Rev Respir Dis. 1984 Apr;129(4):614–618. [PubMed] [Google Scholar]
- Lazraq R., Clavel-Sérès S., David H. L., Roulland-Dussoix D. Conjugative transfer of a shuttle plasmid from Escherichia coli to Mycobacterium smegmatis [corrected]. FEMS Microbiol Lett. 1990 May;57(1-2):135–138. doi: 10.1016/0378-1097(90)90427-r. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L. Genetic basis of tetracycline resistance in urogenital bacteria. Antimicrob Agents Chemother. 1990 Feb;34(2):261–264. doi: 10.1128/aac.34.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Leonard R. B., Briselden A., Schoenknecht F. D., Coyle M. B. Characterization of antibiotic-resistant Corynebacterium striatum strains. J Antimicrob Chemother. 1992 Oct;30(4):463–474. doi: 10.1093/jac/30.4.463. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Pang Y., Riley D. E., Hillier S. L., Berger R. C., Krieger J. N. Detection of Tet M and Tet O tetracycline resistance genes by polymerase chain reaction. Mol Cell Probes. 1993 Oct;7(5):387–393. doi: 10.1006/mcpr.1993.1057. [DOI] [PubMed] [Google Scholar]
- Speer B. S., Shoemaker N. B., Salyers A. A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992 Oct;5(4):387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., Silcox V. A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982 Aug;22(2):186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Lowrie D., Cole S., Colston M. J., Matter L., Schopfer K., Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993 Mar 13;341(8846):647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- Udou T., Mizuguchi Y., Wallace R. J., Jr Patterns and distribution of aminoglycoside-acetylating enzymes in rapidly growing mycobacteria. Am Rev Respir Dis. 1987 Aug;136(2):338–343. doi: 10.1164/ajrccm/136.2.338. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Onyi G. O. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992 Aug;166(2):405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Silcox V. A., Tsukamura M., Nash D. R., Steele L. C., Steingrube V. A., Smith J., Sumter G., Zhang Y. S. Clinical disease, drug susceptibility, and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. J Infect Dis. 1991 Mar;163(3):598–603. doi: 10.1093/infdis/163.3.598. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Tsukamura M., Blacklock Z. M., Silcox V. A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988 Jul;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Bullen M. G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983 Jul-Aug;5(4):657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Heym B., Allen B., Young D., Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992 Aug 13;358(6387):591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]