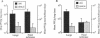Abstract
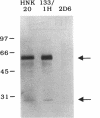
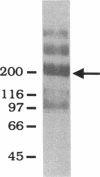
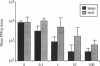
The role of secretory antibody in protection against respiratory syncytial virus (RSV) infection was examined by using monoclonal immunoglobulin A (IgA) antibody for intranasal passive immunization of mice. Eight anti-RSV IgA hybridomas were produced by fusing myeloma cells with lung lymphocytes from RSV-immunized mice. Five IgA antibodies recognized RSV strains of both the A and the B subgroups, and two of these neutralized virus in a plaque reduction assay. Monoclonal IgA antibody HNK20, which bound to F glycoprotein, was most effective, reducing plaques by 50% at a concentration of 0.1 microgram/ml for both subgroup A and subgroup B strains. HNK20 also neutralized all of eight clinical isolates of RSV tested. When delivered intranasally to mice 24 h prior to RSV challenge, HNK20 reduced virus titers in the lungs by nearly 100-fold. Maximal protection occurred at a dose of 0.5 mg/kg of body weight. Significant protection against lung infection was seen when the interval between antibody treatment and challenge was as long as 72 h. HNK20 also decreased virus titers in the nose approximately 10-fold when given 1 h, but not 24 h, before challenge. When mice were treated with HNK20 intranasally 3 days after challenge, viral titers were reduced in the lungs but not the nose. The results indicate that topical application of relatively small amounts of monoclonal IgA can protect against both upper and lower respiratory tract infections caused by RSV.
Full text
PDF

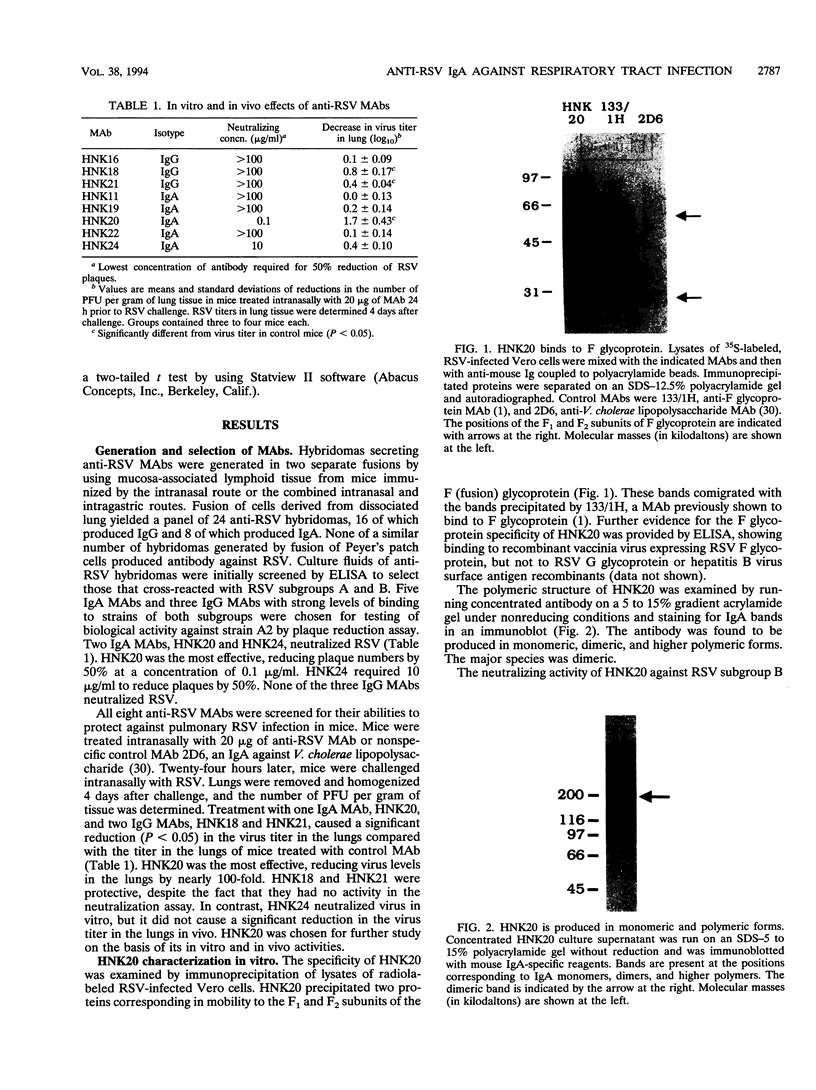
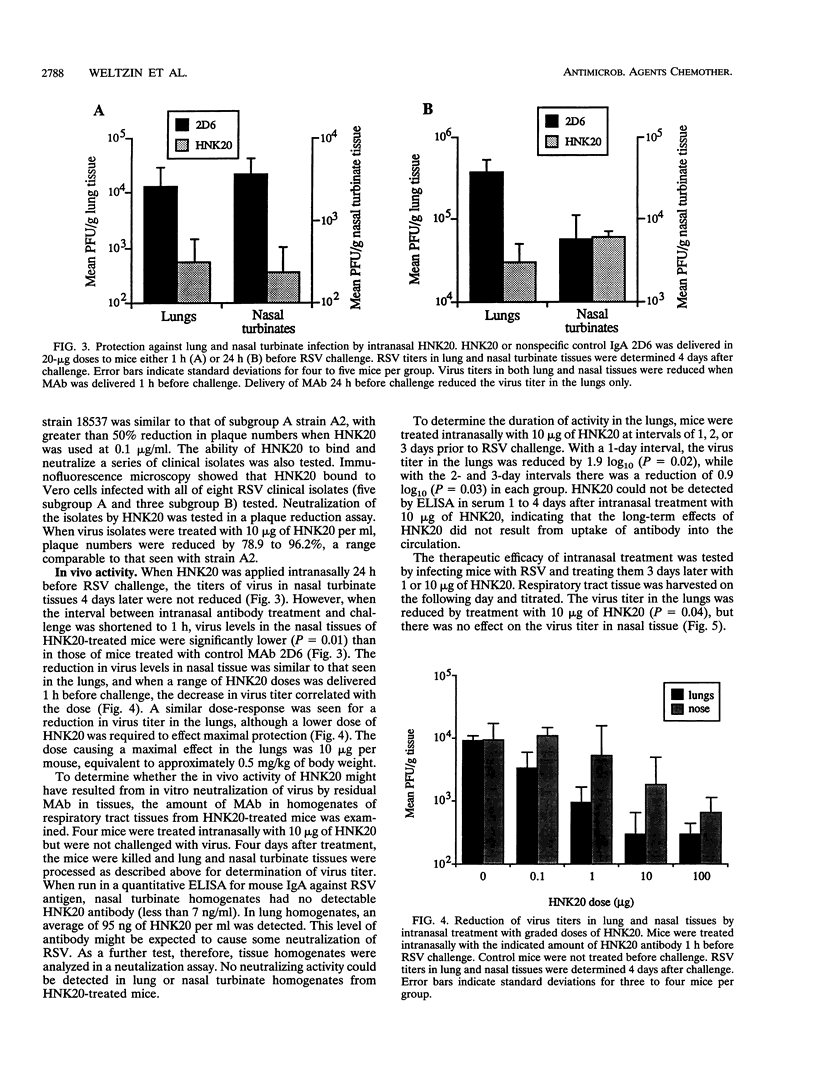
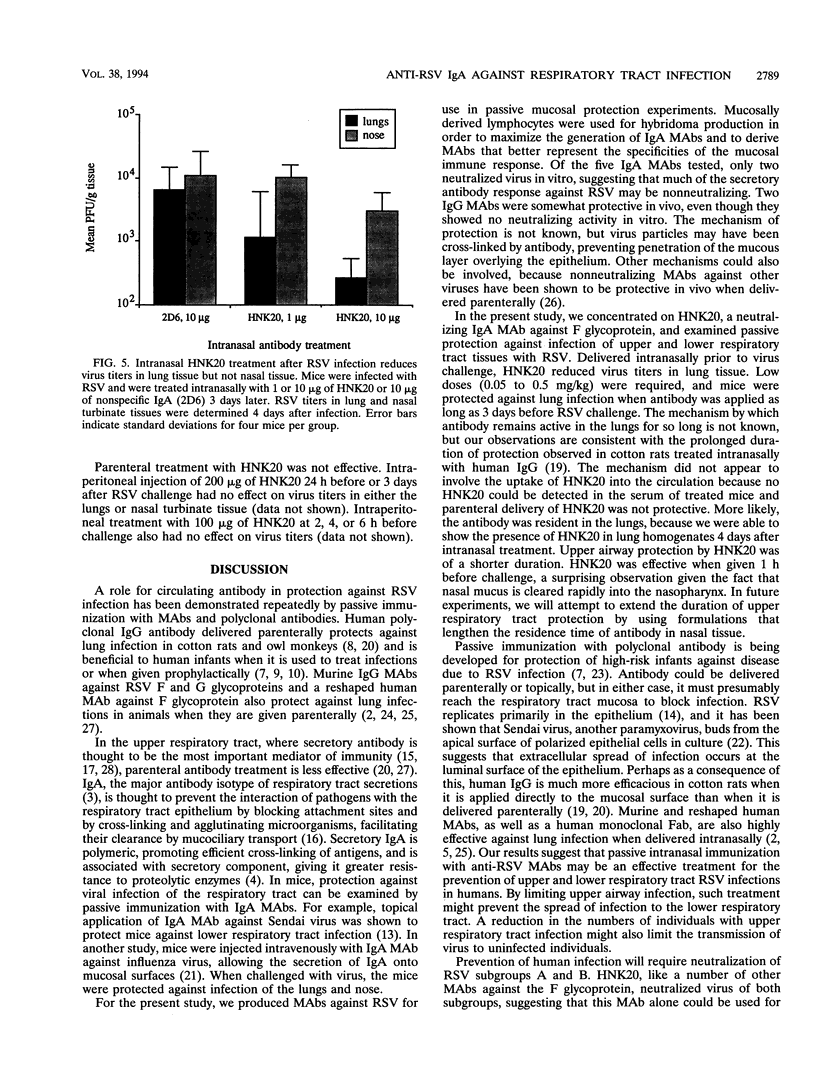


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Bingham P., Hierholzer J. C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988 Nov;62(11):4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Newcomb R. W., Ishizaka K. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest. 1970 Jul;49(7):1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. E., Jr, Murphy B. R., Chanock R. M., Williamson R. A., Barbas C. F., 3rd, Burton D. R. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Paredes A., Allison J. E., Taber L. H., Frank A. L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- Groothuis J. R., Simoes E. A., Levin M. J., Hall C. B., Long C. E., Rodriguez W. J., Arrobio J., Meissner H. C., Fulton D. R., Welliver R. C. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993 Nov 18;329(21):1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., Prince G. A., Horswood R. L., London W. J., Murphy B. R., Walsh E. E., Fischer G. W., Weisman L. E., Baron P. A., Chanock R. M. Studies of passive immunotherapy for infections of respiratory syncytial virus in the respiratory tract of a primate model. J Infect Dis. 1985 Nov;152(5):1083–1087. doi: 10.1093/infdis/152.5.1083. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., Prince G. A., London W. T., Baron P. A., Brown R., Chanock R. M. Topically administered immunoglobulin reduces pulmonary respiratory syncytial virus shedding in owl monkeys. Antimicrob Agents Chemother. 1988 Aug;32(8):1269–1270. doi: 10.1128/aac.32.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., Rodriguez W., Kim H. W., Brandt C. D., Parrott R. H., Burch B., Prince G. A., Baron P. A., Fink R. J., Reaman G. Intravenous immunoglobulin treatment of respiratory syncytial virus infections in infants and young children. Antimicrob Agents Chemother. 1987 Dec;31(12):1882–1886. doi: 10.1128/aac.31.12.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Weltzin R., Soman G., Georgakopoulos K. M., Houle D. M., Monath T. P. Oral administration of polymeric immunoglobulin A prevents colonization with Vibrio cholerae in neonatal mice. Infect Immun. 1994 Mar;62(3):887–891. doi: 10.1128/iai.62.3.887-891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec M. B., Lamm M. E., Lyn D., Portner A., Nedrud J. G. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992 Apr;23(1-2):1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- Mazanec M. B., Nedrud J. G., Lamm M. E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987 Aug;61(8):2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Masters H. B., Orr I., Chao R. K., Barkin R. M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978 Jul;138(1):24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- McNabb P. C., Tomasi T. B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- Mills J., 5th, Van Kirk J. E., Wright P. F., Chanock R. M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971 Jul;107(1):123–130. [PubMed] [Google Scholar]
- Piazza F. M., Johnson S. A., Ottolini M. G., Schmidt H. J., Darnell M. E., Hemming V. G., Prince G. A. Immunotherapy of respiratory syncytial virus infection in cotton rats (Sigmodon fulviventer) using IgG in a small-particle aerosol. J Infect Dis. 1992 Dec;166(6):1422–1424. doi: 10.1093/infdis/166.6.1422. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Hemming V. G., Horswood R. L., Baron P. A., Chanock R. M. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. 1987 Jun;61(6):1851–1854. doi: 10.1128/jvi.61.6.1851-1854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Hemming V. G., Horswood R. L., Chanock R. M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985 Oct;3(3):193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R., Leszcynski J., Pena-Cruz V., Ferren-Gardner C., Anderson R., Hemming V. G., Walsh E. E., Burns J., McIntosh K., Gonin R. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992 Mar;165(3):456–463. doi: 10.1093/infdis/165.3.456. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Tempest P. R., Bremner P., Lambert M., Taylor G., Furze J. M., Carr F. J., Harris W. J. Reshaping a human monoclonal antibody to inhibit human respiratory syncytial virus infection in vivo. Biotechnology (N Y) 1991 Mar;9(3):266–271. doi: 10.1038/nbt0391-266. [DOI] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. J., Robinson B. S., Pringle C. R., Tyrrell D. A. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine. 1990 Jun;8(3):231–236. doi: 10.1016/0264-410x(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Weltzin R., Lucia-Jandris P., Michetti P., Fields B. N., Kraehenbuhl J. P., Neutra M. R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989 May;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]