Abstract
We examined how cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates heterogeneous CD4+ T cell responses by using experimental autoimmune encephalomyelitis (EAE), a CD4+ T cell-mediated disease that is subject to regulation by CTLA-4. Disease incidence and severity were used as measures of in vivo CD4+ T cell responses. The frequency, cytokine production, and reactivity of primed T cells were determined from animals immunized with proteolipid protein (PLP)-139–151 (disease agonist), PLP-Q (disease antagonist), or both peptides, and treated with control or anti-CTLA-4 antibody to analyze the responding population. CTLA-4 blockade exacerbated disease in PLP-139–151-primed animals and overcame disease antagonism in coimmunized animals, but did not permit disease induction in PLP-Q-primed animals. Experimental autoimmune encephalomyelitis enhancement was associated with increased frequencies of cytokine-producing cells and increased ratios of IFN-γ to IL-4 secretors responsive to PLP-139–151. Priming with PLP-Q elicited IL-4 and IL-2, but not IFN-γ secretors cross-reactive with PLP-139–151. Strikingly, CTLA-4 blockade was found to decrease rather than increase the frequencies of cross-reactive IL-4 and IL-2 secretors. Thus, CTLA-4 engagement limits the size, but increases the breadth, of reactivity of a primed pool of CD4+ T cells, consequently regulating its function.
T cell activation is regulated by T cell antigen receptor (TCR)/peptide/MHC interactions and engagement of the costimulatory molecules CD28 and CTLA-4 [cytotoxic T lymphocyte antigen-4 (CD152)] with B7.1 (CD80) or B7.2 (CD86) (1). The TCR determines the potency of peptide/MHC complexes encountered by a T cell via the frequency and duration of TCR/peptide/MHC interactions (2). CD28 provides costimulatory signals that enhance and sustain IL-2 secretion as well as proliferation (3). CTLA-4 is an inhibitory costimulation molecule that restricts IL-2 production and cell cycle progression (4–7). The ctla-4 null mutation results in polyclonal T cell activation and proliferation that is initiated by CD4+ T cells (8–10). These and other data have led to the recognition of CTLA-4 as an important regulator of CD4+ T cell responses.
We have proposed a model in which the higher avidity of CTLA-4 for B7 as compared with CD28 (11) results in inhibition of activating costimulation when B7 levels are low on antigen-presenting cells (APC). When B7 levels are high on APCs, CTLA-4 becomes limiting and CD28/B7-mediated costimulation becomes dominant. The result is cytokine secretion and proliferation. However, activation results in increased surface expression of CTLA-4 that may reach levels sufficient to antagonize activating signals and terminate the T cell response. This model also implies that CTLA-4 signals antagonize TCR signals to set a threshold for the potency or frequency of TCR ligation necessary for CD4+ T cell activation. Thus, the integration of stimulatory CD28/B7 and inhibitory CTLA-4/B7 engagements, with the quantity and/or quality of TCR/peptide/MHC interactions, dictates the biological outcome of a CD4+ T cell encounter with antigen (1, 12, 13).
In this study, experimental autoimmune encephalitis (EAE) was used as a model system to evaluate the contribution of CTLA-4 to the priming of a heterogeneous pool of CD4+ T cells. Immunization of SJL/J mice with the myelin proteolipid protein (PLP)-derived peptide 139–151 in complete Freund's adjuvant primes a diverse pool of antigen-specific T helper 1 (Th1) CD4+ T cells that mediate a quantifiable disease upon encounter of self-antigen in the central nervous system (14–16). Previous studies demonstrated that blockade of CTLA-4 during priming exacerbates clinical and histologic disease (17–19). This suggests that CTLA-4 regulates CD4+ T cell responses under inflammatory conditions when activating TCR and CD28 signals are thought to overwhelm inhibitory CTLA-4 signals. By elucidating a mechanism for anti-CTLA-4-mediated disease exacerbation at the level of the primed population of CD4+ T cells, we hoped to better understand the role of CTLA-4 in regulating polyclonal CD4+ T cell responses in vivo.
This study also used a previously characterized altered peptide ligand of PLP-139–151. PLP-Q is mutated at a critical TCR contact residue (W144Q) (20) and antagonizes disease induction in animals coimmunized with PLP-Q and PLP-139–151. This is thought to occur as a result of priming by PLP-Q of a subset of PLP-139–151-reactive cells that differentiate to a T helper 2 (Th2) phenotype (21). These cells rely on alternative TCR contacts and may express TCRs of lower affinity for PLP-139–151 than do Th1 clones restricted to W144 (21, 22). In addition, some fraction of the pool of Th1 responders normally recruited from the T cell repertoire by PLP-139–151 are weakly antagonized by high concentrations of PLP-Q in vitro (20). This may reflect cross-reactive TCR/peptide/MHC interactions of shorter duration (2, 23).
Animals were immunized with PLP-139–151 or PLP-Q, or coimmunized with both peptides and treated with control or anti-CTLA-4 antibody. The effects of CTLA-4 blockade on disease induction and severity were related to changes in the frequency and cytokine production of peptide-reactive CD4+ T cells. As previously reported, CTLA-4 blockade exacerbated disease in PLP-139–151-immunized animals (17, 19). This corresponded with an increased frequency of IFN-γ producing T cells. Priming with PLP-Q alone did not induce disease in control or anti-CTLA-4-treated animals. IFN-γ -producing T cells cross-reactive with PLP-139–151 were absent in both treatment group. CTLA-4 blockade failed to increase the frequency of T cells primed by PLP-Q that produced IL-4 or IL-2 in response to PLP-139–151. In fact, the frequency of cross-reactive T cells decreased in control versus anti-CTLA-4-treated animals. Disease antagonism by PLP-Q in animals coimmunized with both peptides was overcome by CTLA-4 blockade. This correlated with an increased frequency of T cells producing IFN-γ in response to PLP-139–151 compared with control antibody-treated animals. Thus, CTLA-4 regulates the size of a primed pool of CD4+ T cells that can respond to subsequent antigen encounter as well as the overall reactivity. Regulation of these characteristics impacts the function of a primed pool and eventual outcome of a heterogeneous CD4+ T cell response, as exemplified here in the EAE system.
Materials and Methods
Female Mice.
SJL/J (H-2s) mice (4–6 wk old) were purchased from The Jackson Laboratory and housed at the University of California, Berkeley, in accordance with National Institutes of Health-approved procedures and American Association for the Accreditation of Laboratory Animal Care.
Antigens.
PLP-139–151 (HSLGKWLGHPDKF) and PLP-Q (HSLGKQLGHPDKF) were synthesized at the University of California, Berkeley, Cancer Research Laboratory Microchemical Facility by standard fluorenylmethoxycarbonyl synthesis. Peptides were purified by reverse phase HPLC (>99%) and purity was verified by mass spectroscopy.
Antibodies.
Control hamster IgG (560.31) and anti-CTLA-4 antibody (9H10) were grown in a CellMax according to manufacture's instructions (Cellco, Kensington, MD), purified on protein G-Sepharose columns (Boehringer Mannheim), and eluted with 50 mM diethylamine (Sigma). Antibodies were dialyzed against PBS (pH 7.4). Testing was performed by the University of California, San Francisco, Cell Culture Facility to ensure less than 1.0 ng/ml endotoxin.
Immunizations.
SJL/J animals 8–13 weeks of age were used for immunizations. At day 0, animals received a total of 200 μl of peptide in complete Freund's adjuvant containing 400 μg of heat-killed Mycobacterium tuberculosis H37 RA (Difco) at four separate sites on the dorsolateral surface. Peptide doses used were 60 μg of PLP-139–151 and 300 μg or 500 μg of PLP-Q per animal for single immunization experiments. The lower dose of PLP-Q per animal was used for ELISPOT analysis, but both doses were tested for disease induction. Coimmunized animals received 60 μg of PLP-139–151 plus 300 μg of PLP-Q per animal. Antibodies were injected at 100 μg per animal i.p. on days 0, 3, and 6 for disease progression and days 0 and 3 for ELISPOT analysis.
Clinical Disease and Histological Disease Assessment.
Animals were monitored on a daily basis until days 27 to 30 postimmunization. Disease progression was scored on a scale of 0–5 as previously described: 1 = limp tail; 2 = inability to right itself from back; 3 = hind limb paralysis; 4 = front and hind limb paralysis; 5 = moribund/dead. Disease assessment was performed without knowledge of the treatment administered to the individual animals. At the end of the experiment, animals were killed. Brains and spinal cords were removed and inflammatory foci were enumerated without knowledge of the treatment, as previously described (24).
ELISPOT.
Animals were killed at day 8 postimmunization. Inguinal, brachial, and axillary lymph nodes were harvested, pooled from 4 or 5 animals per group, dissociated, and their red blood cells lysed. Single cell suspensions were plated 5 × 105 cells per well in 96-well plates (Millipore) coated with 4 μg/ml anti-mouse IL-4, 4 μg/ml anti-mouse IL-2, or 10 μg/ml anti-mouse IFN-γ (PharMingen) in the presence of the indicated peptide concentrations. At 36 h, plates were washed with PBS/Tween-20 and incubated with 5 μg/ml biotinylated anti-mouse IL-4, IL-2, or IFN-γ antibodies (PharMingen) for 5 h at room temperature. After washing, streptavidin-horseradish peroxidase (Jackson ImmunoResearch) was added (1:1000) for 1 h at room temperature, followed by additional washing and development with the peroxidase substrate 3-amino-9-ethylcarbazole (Sigma). Spots were assessed by using a dissecting microscope.
Flow Cytometry.
Lymph node cells used in ELISPOT assays were analyzed to ensure equivalent numbers of CD4+ T cells and APC were present in treatment groups. Day 8 draining lymph node cells (1 × 106) were incubated with the anti-Fcγ receptor antibody 24G2 (Allison Lab, University of California, Berkeley). After Fcγ receptor-blocking, cells were stained with anti-mouse TCRβ-PE (H57; PharMingen; PE, phycoerythrin), anti-mouse B220-FITC (Allison Lab), and anti-mouse CD4-TriColor (Caltag, South San Francisco, CA) for 30 min at 4°C. The cells were analyzed on an XL (Coulter) using elite software. At least 20,000 events were collected within a live gate. Neither the total lymph node cell number nor the percentages of CD4+, B220 +, and TCRβ+ cells varied within experiments as a result of the different immunization regimes (data not shown).
Statistical Analysis.
The statistical significance of disease incidence was calculated by the Fisher exact test. Mann–Whitney tests were performed for the mean peak clinical scores by using prism software (GraphPad Software, San Diego).
Results
Priming with PLP-Q Does Not Induce EAE if CTLA-4 Is Blocked.
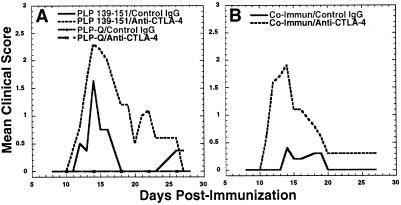
As previously reported, blockade of CTLA-4 during immunization with PLP-139–151 resulted in 100% disease incidence and exacerbated disease (Fig. 1A and Table 1) (17, 19). The ability of anti-CTLA-4 treatment to convert the disease-antagonizing peptide PLP-Q to a disease agonist was assessed for two reasons. First, lowering an activation threshold set by CTLA-4 blockade might be expected to prime a greater frequency of disease-inducing Th1 CD4+ T cells specific to PLP-139–151, but weakly reactive to PLP-Q. Second, if T helper differentiation is proportional to TCR signal strength (25), decreasing CTLA-4 antagonism of TCR signals may be expected to skew PLP-Q-primed CD4+ T cells cross-reactive to PLP-139–151 to a disease-inducing Th1 phenotype instead of a protective Th2 phenotype (21). We found that priming with PLP-Q at 300 or 500 μg per animal failed to induce disease whether or not CTLA-4 was blocked (Fig. 1A and data not shown). The same results were obtained if animals were primed with 500 μg per animal of the antagonistic altered peptide PLP-LR (W144L, H147R) (20) (data not shown). These data do not seem to support the threshold scenarios. However, disease data alone cannot rule out that one or both of the above predictions were correct but occurred at a magnitude too small to induce disease.
Figure 1.
Blockade of CTLA-4 exacerbates EAE and overcomes altered peptide ligand-mediated disease inhibition, but fails to convert the altered peptide ligand to a disease agonist. (A) Blockade of CTLA-4 exacerbates disease in animals primed with PLP-139–151 but fails to induce disease in PLP-Q immunized animals. (B) Blockade of CTLA-4 overcomes disease antagonism by PLP-Q in PLP-Q plus PLP-139–151 coimmunized animals. Animals were immunized with the indicated peptides in complete Freund's adjuvant and treated with control or anti-CTLA-4 antibody, as described in the Materials and Methods. The data for PLP-Q are from animals immunized with 300 μg of peptide. Clinical disease was assessed daily. These data are representative of four separate experiments.
Table 1.
Anti-CTLA-4 treatment overcomes disease antagonism by PLP-Q
| Peptide | Antibody | Disease incidence | Mean peak clinical score | Mean day of onset | Mean total foci |
|---|---|---|---|---|---|
| PLP-139–151 | Control IgG | 18/23 | 1.8 ± 0.4* | 11.8 ± 1.5 | 43.9 ± 10.7 |
| PLP-139–151 | Anti-CTLA-4 | 23/23 | 2.5 ± 0.5 | 11.5 ± 1.1 | 91.3 ± 18.3 |
| 5:1 PLPQ:PLP-139–151 | Control IgG | 8/25† | 1.3 ± 0.3‡§ | 12.4 ± 1.6 | 19.4 ± 7.0 |
| 5:1 PLPQ:PLP-139–151 | Anti-CTLA-4 | 20/25 | 2.0 ± 0.7 | 12.5 ± 2.1 | 38.2 ± 8.5 |
Data are cumulative from four separate experiments, except mean total foci, which are from two of the four experiments. Mean peak clinical score and day of onset are presented for those animals in which disease occurred (score ≥ 1.0) ± SD. Mean total foci are presented ± SE (n = 13–15 animals per treatment condition). Individual experiments consisted of 5–10 animals per group.
P < 0.0003 compared with PLP-139–151/anti-CTLA-4.
P = 0.0013 compared with coimmunization/anti-CTLA-4.
P < 0.0213 compared with coimmunization/anti-CTLA-4.
P < 0.006 compared with PLP-139–151/control IgG antibody.
CTLA-4 Blockade Overcomes PLP-Q Protection in Coimmunization Experiments.
We next determined whether CTLA-4 blockade could overcome PLP-Q-mediated disease antagonism in animals immunized with both PLP-Q and PLP-139–151. As predicted (21), coimmunization with PLP-Q and PLP-139–151 in the presence of control hamster antibody resulted in a lower disease incidence (32%) compared with immunization with PLP139–151 and control antibody only (78%) (Table 1). Of the animals that did exhibit clinical disease in the coimmunized groups, the mean peak disease severity was lower than the group immunized with PLP-139–151 alone. Blockade of CTLA-4 during priming reversed disease inhibition by PLP-Q in the coimmunized groups, resulting in a disease incidence (80%) and peak disease severity similar to that of the animals immunized with control antibody and PLP-139–151 only. The histologic data show the same trend (Table 1). Fig. 1B represents a typical disease course of four experiments, illustrating that CTLA-4 blockade can overcome PLP-Q-mediated disease inhibition.
CTLA-4 Blockade Results in an Increase in Peptide-Specific Responders.
To determine a mechanism at the cellular level for the disease data presented in Fig. 1 and Table 1, the frequency of primed CD4+ T cells capable of secreting cytokines on subsequent antigen encounter, as occurs in the central nervous system for disease induction, was assessed in vitro via ELISPOT. IL-10 production was not detected from PLP-Q or coimmunized animals (data not shown). IFN-γ and IL-4 were assessed as indicators of Th1 and Th2 responders, respectively.
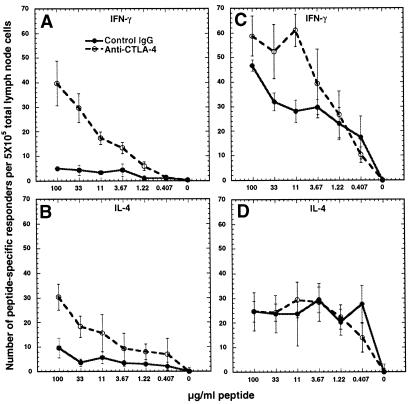
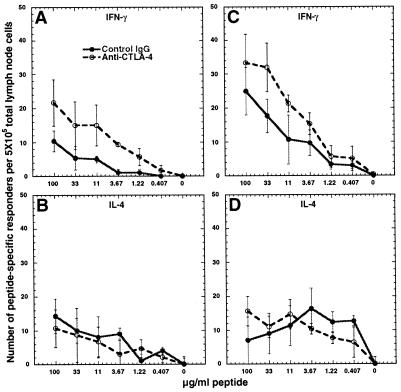
An increase in the frequency of IFN-γ- and IL-4-secreting cells was observed from mice immunized with PLP-139–151 and treated with anti-CTLA-4, compared with control antibody-treated animals, on restimulation with PLP-139–151 (Fig. 2 A and B). The frequency of IFN-γ, but not IL-4, secreting cells responsive to PLP-Q also increased in animals primed with PLP-Q and treated with anti-CTLA-4 (Fig. 2 C and D). Likewise, an increase in IFN-γ secreting responders specific for PLP-Q or the parental peptide occurred in animals treated with anti-CTLA-4 and immunized with both peptides (Fig. 3 A and C). The number of responders making IL-4 was not affected by CTLA-4 blockade during priming in the coimmunized animals (Fig. 3 B and D).
Figure 2.
Blockade of CTLA-4 during priming results in an increase in responders to the priming peptide. CTLA-4 blockade results in increased IFN-γ- (A) and IL-4- (B) secreting responders to PLP-139–151 in animals immunized with PLP-139–151. Anti-CTLA-4 results in increased IFN-γ- (C) but not IL-4- (D) secreting responders (D) to PLP-Q in PLP-Q-immunized animals. Animals (four or five per group) were immunized with PLP-139–151 or PLP-Q and control (solid lines) or anti-CTLA-4 antibody (dashed lines) as described in Materials and Methods. ELISPOT analysis was performed on day 8. Draining lymph nodes were pooled and cells were stimulated at 5 × 105 cells per well in wells coated with capture antibody for IFN-γ or IL-4, with the indicated concentrations of peptide for 36 h. A and B are representative of four experiments whereas C and D represent two of three experiments (IL-4 responders increased in one experiment). Data points represent the means of triplicate wells. Error bars represent standard deviation. For IL-4, the number of peptide-specific responders represents the numbers of spots in the presence of peptide minus the number of IL-4 secretors in the control wells without peptide.
Figure 3.
Blockade of CTLA-4 increases the frequency of Th1 but not Th2 responders in coimmunized animals. Blockade of CTLA-4 in animals primed with a 5:1 mixture of PLP-Q/PLP-139–151 results in an increase in IFN-γ secretors responding to PLP-139–151 (A) and PLP-Q (C). The frequency of IL-4 responders to PLP-139–151 (B) and PLP-Q (D) is not affected. Experiments were performed as in Fig. 2. These data are representative of four separate experiments.
These data suggest that an increase in the frequency of Th1 responders contributes to disease exacerbation in anti-CTLA-4-treated animals primed with PLP-139–151. A similar mechanism appears to be important in overcoming PLP-Q-mediated disease amelioration in coimmunized animals.
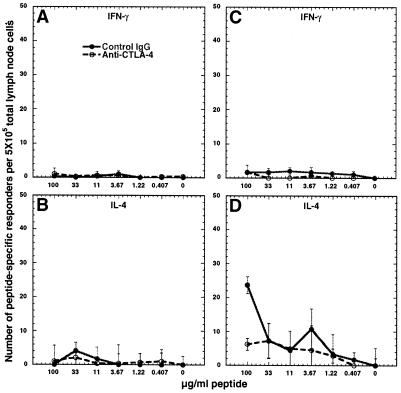
Blockade of CTLA-4 Does Not Allow the Generation of PLP-139–151-Responsive Th1 Cells in PLP-Q-Primed Animals.
Analysis of CD4+ T cells primed in vivo with only PLP-139–151 or PLP-Q was performed to assess the effect of CTLA-4 blockade on the effector function and frequency of responders reactive to both peptides. No cross-reactive IFN-γ producers were detected in animals primed with either peptide (Fig. 4 A and C). Low numbers of responders were detected from mice primed with PLP-139–151 and treated with control or anti-CTLA-4 antibody that secreted IL-4 in response to PLP-Q (Fig. 4B). A population of cross-reactive cells that made IL-4 in response to the parental peptide from PLP-Q-primed animals was observed (Fig. 4D) as expected (21). In two of three experiments, more cells making IL-4 were detected from animals immunized with PLP-Q and control hamster antibody in comparison with those treated with anti-CTLA-4 antibody at the highest concentration of peptide used for stimulation (Fig. 4D). It is important to note that animals were immunized with 5 times as much PLP-Q as PLP-139–151. Greater ligand density may play a role in priming cells with TCRs weakly reactive to a peptide/MHC complex (26). This might partially explain the lower frequency of PLP-Q-responsive IL-4 secretors from PLP-139–151-immunized animals compared with the number of responders to PLP-139–151 from PLP-Q-primed animals (Fig. 4 B and D).
Figure 4.
Blockade of CTLA-4 does not increase the frequency of cross-reactive cells. Anti-CTLA-4 does not result in cross-reactive IFN-γ responders to PLP-Q from PLP-139–151-primed animals (A) or to PLP-139–151 from PLP-Q-primed animals (C). CTLA-4 blockade can result in a decrease in the number of cross-reactive IL-4 responders. (B) Few IL-4 secretors were observed from PLP-139–151-primed animals in response to PLP-Q. Cross-reactive IL-4 secretors were observed from PLP-Q-primed animals in response to PLP-139–151. (D) CTLA-4 blockade resulted in a decreased number of IL-4-secreting cells at the highest concentrations of PLP-139–151 used for detection. Experiments were performed as described in the legend of Fig. 2. These data are representative of three experiments for A, B, and C and two of three experiments for D.
Taken together, these data suggest that disrupting CTLA-4/B7 interactions during priming with PLP-Q does not result in the differentiation of Th1 cells capable of responding to the parental peptide. The failure to increase the numbers of PLP-139–151-reactive Th2 responders in PLP-Q-immunized animals suggests that CTLA-4 blockade does not result in an increase in the number of T cells expressing TCRs reactive to both peptides.
CTLA-4 Blockade Results in a Lower Frequency of Cross-Reactive CD4+ T Cells.
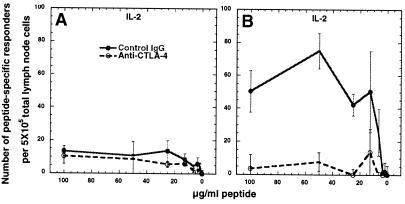
Finally, IL-2 secretion was assessed to further investigate the possibility that reducing a threshold for activation set by CTLA-4 might result in an increase in T cells primed with PLP-Q that respond to the parental peptide. As with IL-4, few cross-reactive IL-2-secreting cells were found to respond to PLP-Q from PLP-139–151-primed animals treated with control antibody or anti-CTLA-4 during priming (Fig. 5A). No consistent differences in the number of IL-2-producing cells reactive to their priming peptide was observed between antibody treatment groups, with only one of three experiments presenting a significant increase when CTLA-4 was blocked (data not shown). Cross-reactive IL-2 secretors were detected in response to PLP-139–151 from animals primed with PLP-Q and treated with control antibody. CTLA-4 blockade resulted in a marked reduction in the number of cross-reactive IL-2 producing cells (Fig. 5B). Preliminary experiments using another altered peptide ligand, PLP-LR, produced similar results (unpublished data).
Figure 5.
The frequency of cross-reactive IL-2 secretors decreases if CTLA-4 is blocked during priming. (A) Few responders are seen from PLP-139–151-primed animals in response to PLP-Q. (B) PLP-Q immunization primes cells that secrete IL-2 in response to PLP-139–151 and the frequency of these responders is reduced if CTLA-4 was blocked during priming. These experiments were performed as in Fig. 2, but with antibodies to mouse IL-2. The data are representative of two and three separate experiments for A and B, respectively.
Consistent with the data regarding IL-4-producing T cells, the IL-2 data demonstrate that CTLA-4 blockade failed to increase the number of cells capable of responding to both PLP-Q and PLP-139–151 within the primed pool of CD4+ T cells. CTLA-4 blockade actually resulted in a decrease in representation of cells within the PLP-Q-primed pool of CD4+ T cells that were cross-reactive to PLP-139–151.
Discussion
While several in vivo systems have been used to demonstrate the significance of CTLA-4 as an inhibitor of T cell responses to antigen, the exact mechanisms are still being elucidated. The initial observation that CTLA-4 blockade greatly enhanced anti-tumor immunity was suggestive of an effect on the threshold of activating signals necessary to elicit a T cell response (27). This may represent a scenario where TCR occupancy is weak or infrequent and B7 levels are limiting. However, the situation is different when antigen-specific T cells are primed by antigen in strong adjuvant. This is presumed to result in the induction of high levels of peptide/MHC complexes and B7 expression on professional APC and facilitate T cell responses. It is not yet clear what effect CTLA-4 might have in shaping CD4+ T cell responses under these conditions.
EAE represents a useful in vivo system for studying the role of CTLA-4 during priming of a heterogeneous pool of antigen-specific CD4+ T cells when B7 levels are not limiting. Anti-CTLA-4 treatment exacerbates clinical and histologic disease (17–19). This has been attributed to enhanced expansion of CD4+ T cells normally primed by encephalitogenic peptides, or to a larger responding pool due to the recruitment of CD4+ T cells stimulated by TCR/peptide/MHC interactions that would have otherwise been too weak to allow for activation (19). These possibilities are not mutually exclusive.
We found that blockade of CTLA-4 during priming with the disease-inducing peptide PLP-139–151, the disease-antagonist PLP-Q, or a mixture of both peptides resulted in an increased frequency of cytokine-producing cells specific to the peptides with which they were activated in vivo, compared with control antibody-treated animals. This indicates that CTLA-4 engagement limits the size of a primed pool of CD4+ T cells that responds to subsequent encounter with antigen.
Disease exacerbation by CTLA-4 blockade in PLP-139–151-immunized animals corresponded with an increase in both IFN-γ- and IL-4-producing cells. However, the degree of increase in IFN-γ-producing cells, and thus the ratio of Th1 to Th2 cells, was consistently greater. This explains previous findings that CTLA-4 blockade during EAE induction results in increased proinflammatory cytokines (17, 18). The reversal of PLP-Q-mediated disease antagonism by CTLA-4 blockade in animals coimmunized with PLP-Q and the parental peptide also corresponded with an increase in IFN-γ-producing cells responsive to subsequent encounter of PLP-139–151. The frequency of IL-4-producing cells was not altered, compared with control antibody-treated animals. Therefore, the ratio of Th1 to Th2 cells responsive to PLP-139–151 was also increased in coimmunized animals. Given that the adoptive transfer of Th1 cells reactive to PLP-139–151 is sufficient to induce disease in SJL/J mice (14, 15), a preferential increase in Th1 responders appears to be the mechanism whereby CTLA-4 blockade exacerbates EAE in animals primed with the PLP-139–151 and overcomes disease antagonism in coimmunized animals. These findings are not consistent with a model in which CTLA-4 engagement limits Th2 and promotes Th1 responses (28). Rather, it seems that the contribution of CTLA-4 to Th differentiation is context dependent. These data demonstrate that CTLA-4 can regulate the overall function of a primed pool of CD4+ T cells, in part, by regulating the size of the pool and the frequency of Th1 vs. Th2 responders.
Blockade of CTLA-4 failed to induce disease in PLP-Q-primed animals. This was because of a failure to prime Th1 cells that could respond to the native peptide. As expected from previous work (21), Th2 cells responsive to the parental peptide were observed in control antibody-treated animals and in anti-CTLA-4-treated animals. Thus, there was no evidence for the differentiation of cross-reactive cells to a Th1 phenotype, instead of a Th2 phenotype, as a result of CTLA-4 blockade. Nor was there any indication that Th1 cells were recruited to the responding pool that were reactive to the PLP-139–151 but weakly reactive to PLP-Q. Further, the population of cross-reactive IL-4-producing cells did not increase if CTLA-4 was blocked during priming. Rather, this population seemed to be slightly decreased compared with the control antibody-treated pool. These data are not consistent with the idea that blockade of CTLA-4 recruits T cells to the primed pool that express TCRs of low affinity for the priming peptide/MHC.
The finding that the frequency of cross-reactive IL-2-producing cells decreased if CTLA-4 was blocked during priming with PLP-Q indicates that cross-reactive cells have decreased representation within the pool. The frequency of cross-reactive CD4+ T cells cannot be directly measured in animals coimmunized with both peptides. However, it can be inferred from the decrease of cross-reactive cells in PLP-Q-immunized animals treated with anti-CTLA-4 that there is also a decreased frequency of cross-reactive cells in the coimmunized animals when CTLA-4 is blocked. This suggests that blockade of CTLA-4 in coimmunized animals resulted in less overlap of reactivity between the PLP-139–151 and PLP-Q-specific subsets of responders. Therefore, reversal of PLP-Q-mediated antagonism in anti-CTLA-4-treated animals was a result of a decreased frequency of cross-reactive cells capable of antagonizing disease induction and an increased frequency of Th1 cells that are not cross-reactive. Thus, CTLA-4 regulates the function of the responding population, in part, by regulating the breadth of reactivity.
Overall, the data presented here are consistent with the idea that CTLA-4 engagement during priming regulates the size of a responding pool of CD4+ T cells by regulating their expansive capacity. This is not surprising, given that CTLA-4 blockade results in increased expansion of monoclonal and oligoclonal CD4+ T cells responding to cognate peptide antigen or superantigen, respectively (29, 30). However, changes in the overall pattern of reactivity due to CTLA-4 blockade suggest that regulation of expansion may not be uniform for all of the responding clones. We have demonstrated that CTLA-4 most dramatically restricts antigen-specific proliferative responses of naive TCR transgenic CD4+ T cells at the highest levels of TCR occupancy in vitro (31). Differential regulation of the expansive capacity of CD4+ T cells by CTLA-4, depending on TCR engagement, could greatly affect the size and clonal representation of the responding population. Blockade of CTLA-4 during priming could give a selective advantage to those cells stimulated by high TCR affinity. This would favor their expansion and result in the decreased representation of responders that are stimulated by low TCR affinity. CTLA-4 blockade would thus increase the extent or rate to which antigen-driven selection promotes the evolution of a “best-fit” population for a pool of T cell responders (32–34). Conversely, CTLA-4 engagement may serve to broaden clonal representation and reactivity of a primed population of heterogeneous T cells by preferentially regulating the best-fit responders.
It is not clear whether broadened reactivity of the responding population of T cells by CTLA-4 engagement is an evolutionary strategy to fully exploit or tightly regulate the potential of the TCR repertoire. Greater breadth may allow for flexibility in responding to mutating pathogenic epitopes. However, diverse clonal representation in an antigen-responsive pool may regulate the magnitude or duration of a response. It has been reported that antigen-specific CD4+ T cell populations with broad reactivity are susceptible to suppression of inflammatory responses by clones with weak TCR/peptide/MHC interactions, including EAE antagonism by PLP-Q (21), or susceptibility to Plasmodium falciparum in malaria-endemic areas (35). A lower frequency of best-fit responders, and a smaller pool size, may make a primed population more susceptible, as a whole, to the cytokine contributions of regulatory populations.
In sum, the data presented here demonstrate that CTLA-4 plays a role in regulating the size and reactivity, and thus the overall function, of a primed pool of CD4+ T cells. Perhaps the most striking finding was that CTLA-4 blockade resulted in a decreased representation of T cells cross-reactive to related peptides. This suggests a previously unrecognized role for CTLA-4 in contributing to the breadth of reactivity of a primed population, possibly by preferentially constraining the best-fit population. Further studies are needed to determine how these findings relate to the clonal representation and overall TCR affinity of antigen-specific CD4+ T cells within a primed pool.
Acknowledgments
We thank Ana Anderson, Cynthia Chambers, Jackson Egen, Andy Hurwitz, and Tim Sullivan for critically reading the manuscript and/or helpful discussions. We also thank Stan Grell for antibody production and purification, and Mario Andreou for technical assistance. This work was supported by grants from the National Cancer Institute (CA 09041 and CA 40041 to J.P.A.) and the National Multiple Sclerosis Society (RG3055 to R.A.S.). J.P.A. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- PLP
proteolipid protein
- APC
antigen-presenting cells
- TCR
T cell antigen receptor
- Th1
T helper 1
- Th2
T helper 2
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220423597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220423597
References
- 1.Chambers C A, Krummel M F, Boitel B, Hurwitz A A, Sullivan T J, Fournier S, Cassell D, Brunner M, Allison J P. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 2.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 3.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 4.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 5.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krummel M F, Allison J P. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner M C, Chambers C A, Chan F, Hanke J, Winoto A, Allison J P. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 8.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 9.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Chambers C A, Cado D, Truong T, Allison J P. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene J L, Leytze G M, Emswiler J, Peach R, Bajorath J, Cosand W, Linsley P S. J Biol Chem. 1996;271:26762–26771. doi: 10.1074/jbc.271.43.26762. [DOI] [PubMed] [Google Scholar]
- 12.Chambers C A, Allison J P. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 13.Chambers C A, Allison J P. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 14.Kuchroo V K, Sobel R A, Laning J C, Marin C, Greenfield E, Dorf M E, Lees M B. J Immunol. 1992;148:3776–3782. [PubMed] [Google Scholar]
- 15.Kuchroo V K, Martin C A, Greer J M, Ju S T, Sobel R A, Dorf M E. J Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 16.Sobel R A, Greer J M, Kuchroo V K. Neurochem Res. 1994;19:915–921. doi: 10.1007/BF00968701. [DOI] [PubMed] [Google Scholar]
- 17.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 19.Hurwitz A A, Sullivan T J, Krummel M F, Sobel R A, Allison J P. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 20.Kuchroo V K, Greer J M, Kaul D, Ishioka G, Franco A, Sette A, Sobel R A, Lees M B. J Immunol. 1994;153:3326–3336. [PubMed] [Google Scholar]
- 21.Nicholson L B, Greer J M, Sobel R A, Lees M B, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu Das M, Nicholson L B, Greer J M, Kuchroo V K. J Exp Med. 1997;186:867–876. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons D S, Lieberman S A, Hampl J, Boniface J J, Chien Y, Berg L J, Davis M M. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 24.Sobel R A, Kuchroo V K. J Immunol. 1992;149:1444–1451. [PubMed] [Google Scholar]
- 25.Constant S L, Bottomly K. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 26.Rees W, Bender J, Teague T K, Kedl R M, Crawford F, Marrack P, Kappler J. Proc Natl Acad Sci USA. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach D, Krummel M, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 28.Oosterwegel M A, Mandelbrot D A, Boyd S D, Lorsbach R B, Jarrett D Y, Abbas A K, Sharpe A H. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 29.Kearney E R, Walunas T L, Karr R W, Morton P A, Loh D Y, Bluestone J A, Jenkins M K. J Immunol. 1995;155:1033–1036. [PubMed] [Google Scholar]
- 30.Walunas T L, Bluestone J A. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 31.Chambers C A, Kuhns M S, Allison J P. Proc Natl Acad Sci USA. 1999;96:8603–8608. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busch D H, Pamer E G. J Exp Med. 1999;189:701–709. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savage P A, Boniface J J, Davis M M. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 34.McHeyzer-Williams L J, Panus J F, Mikszta J A, McHeyzer-Williams M G. J Exp Med. 1999;189:1823–1837. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plebanski M, Flanagan K L, Lee E A M, Reece W H H, Hart K, Gelder C, Gillespie G, Pinder M, Hill A V S. Immunity. 1999;10:651–660. doi: 10.1016/s1074-7613(00)80064-3. [DOI] [PubMed] [Google Scholar]