Abstract
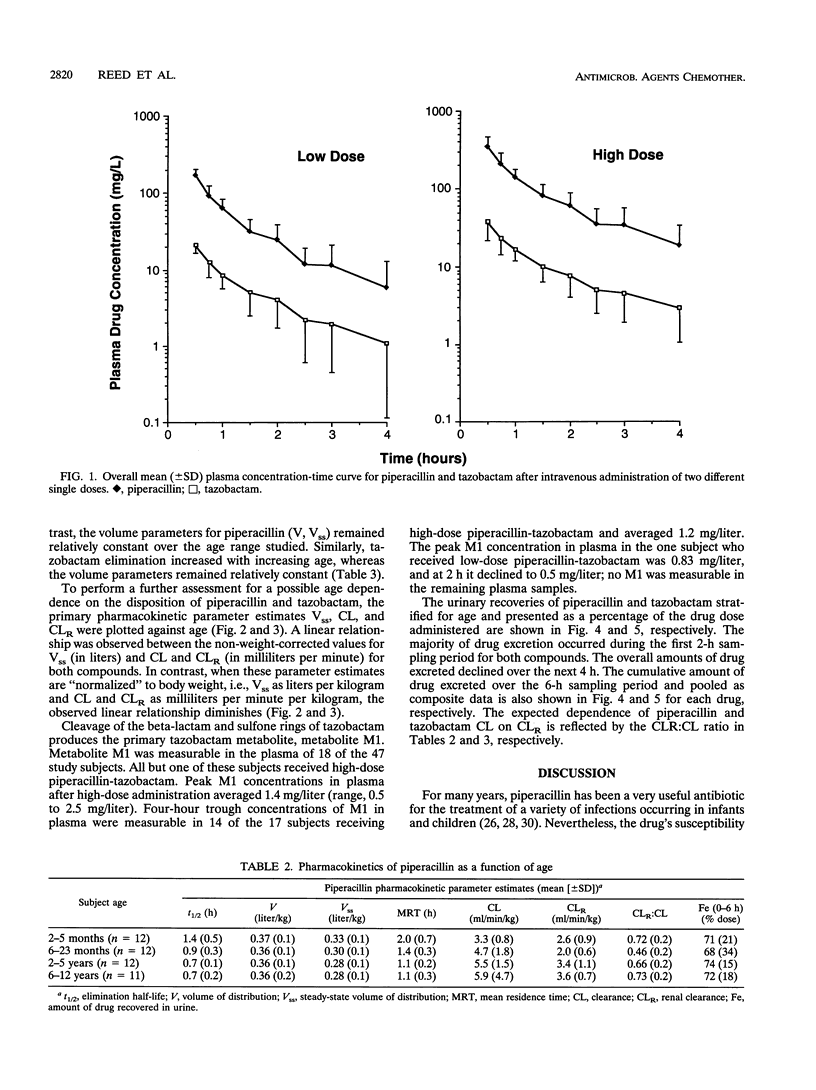
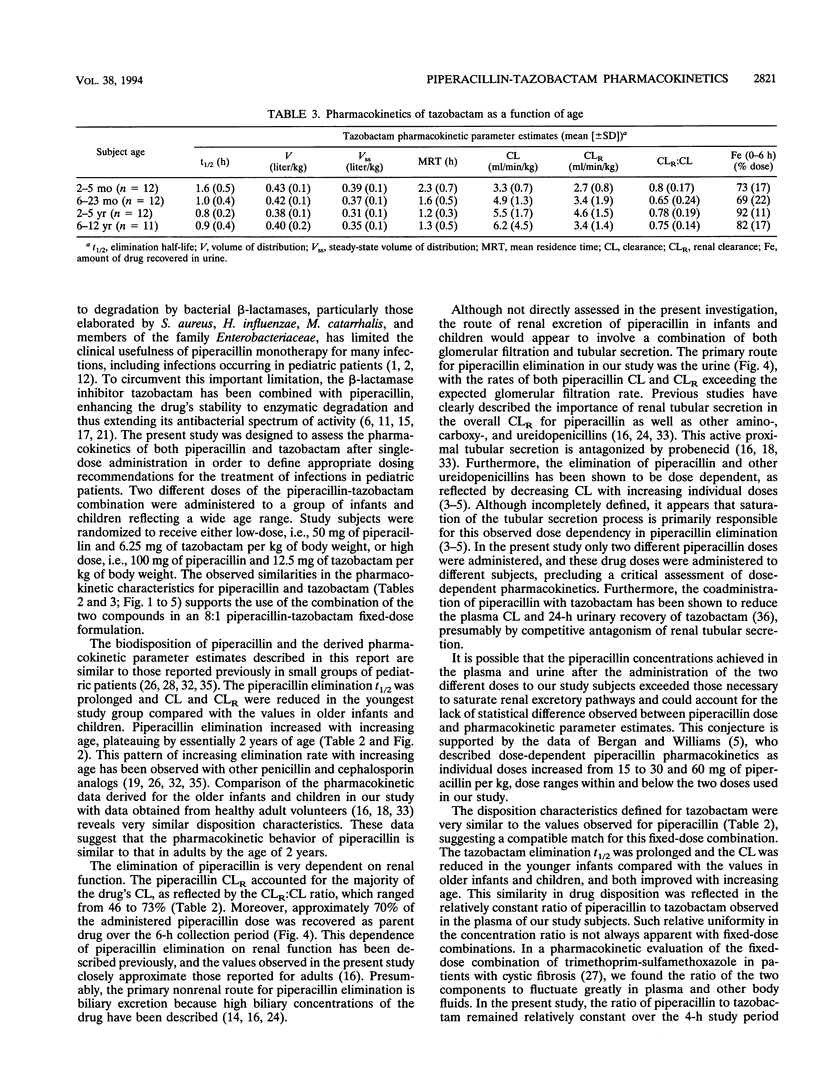
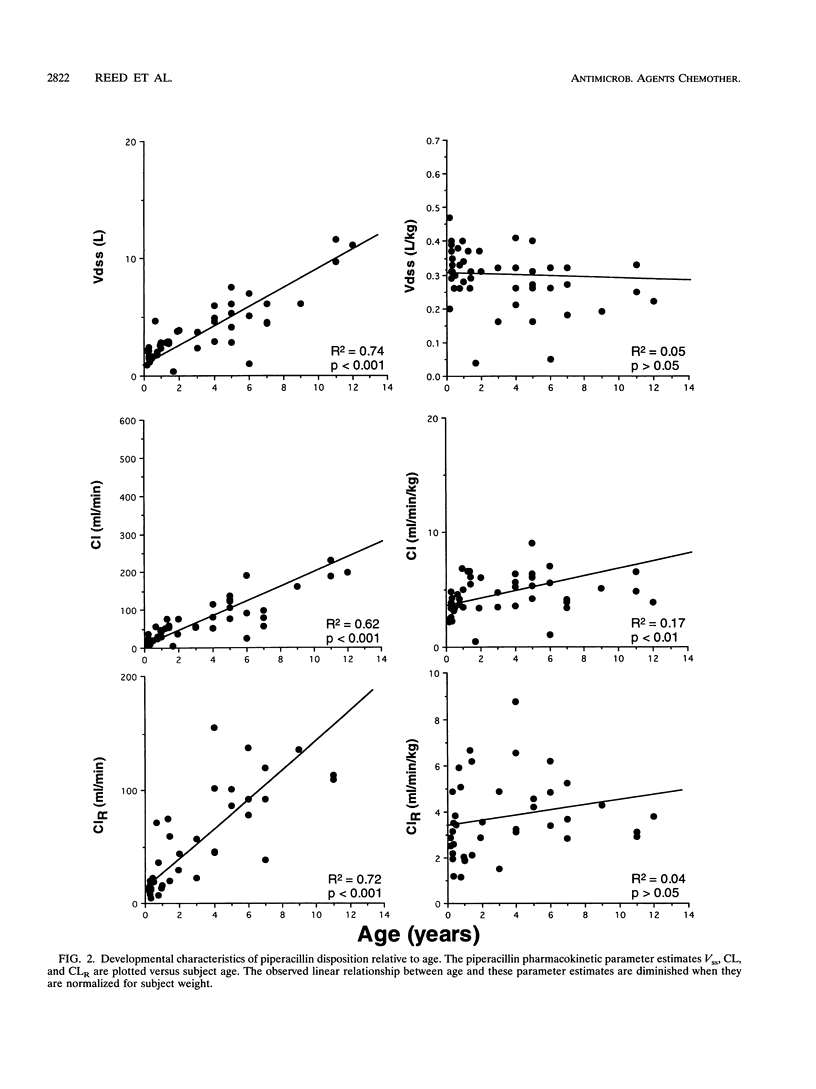
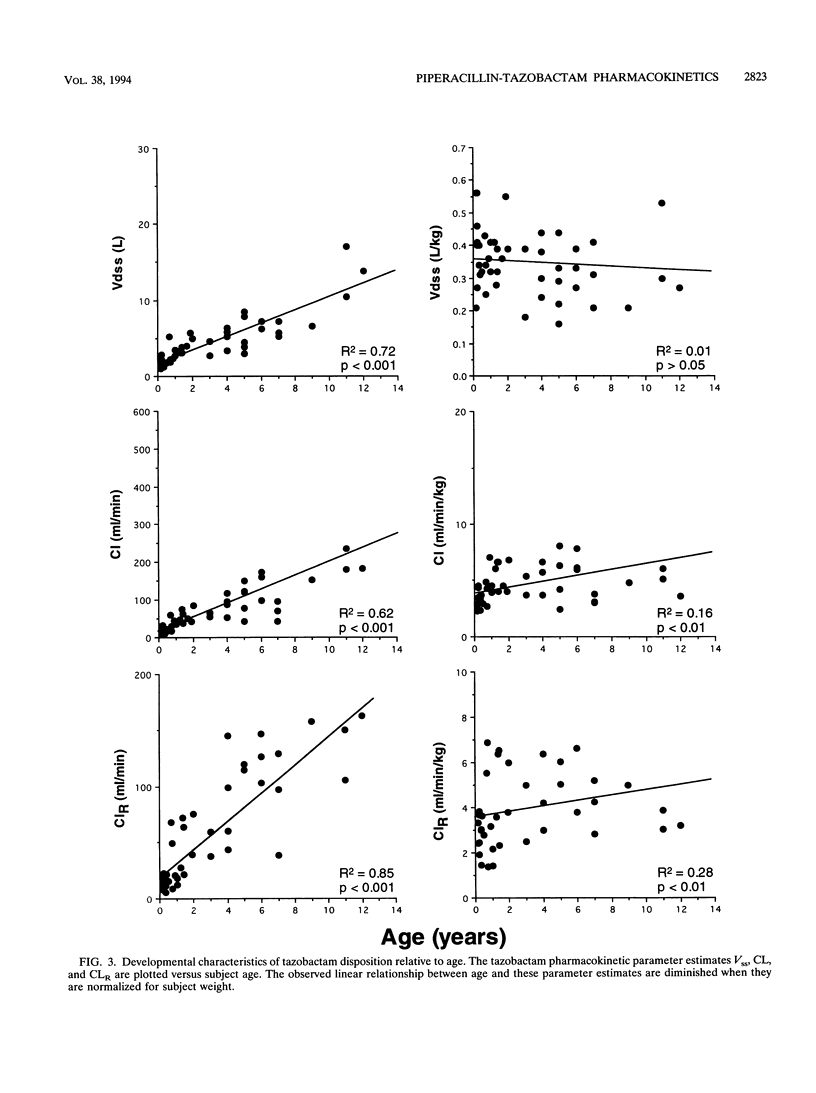
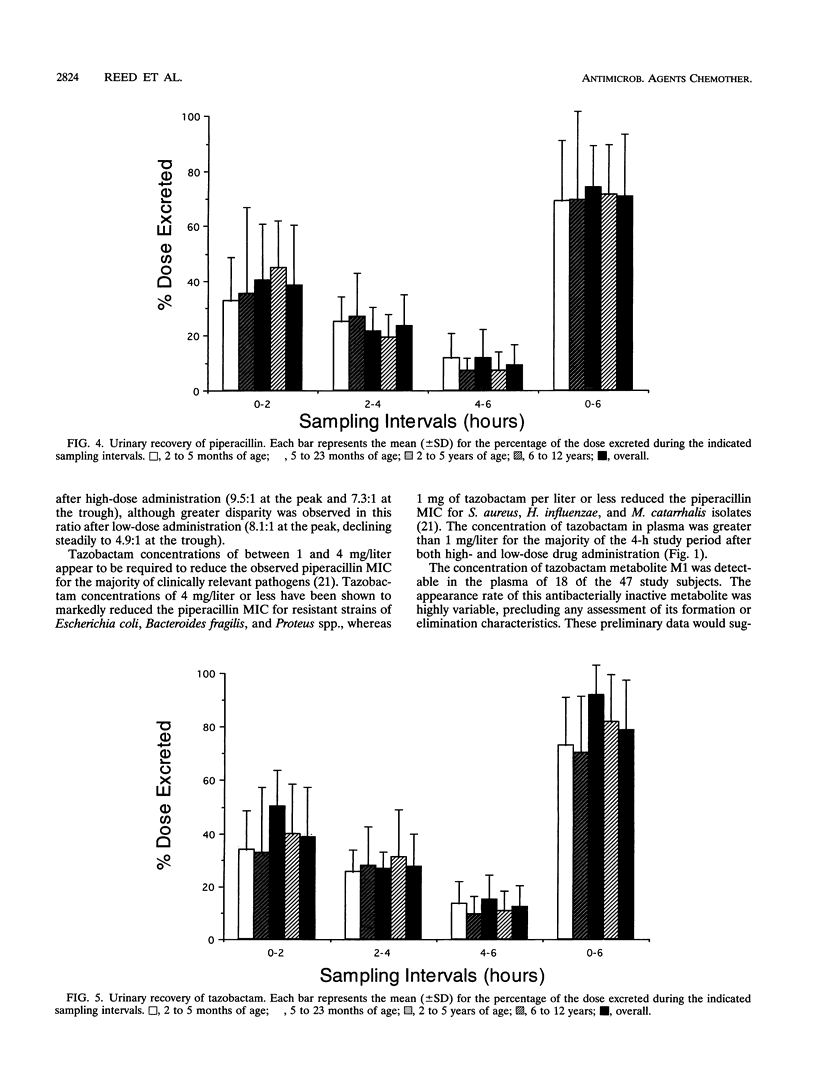
The pharmacokinetics of piperacillin and tazobactam were assessed after single-dose administration to 47 infants and children. Study subjects ranging in age from 2 months to 12 years were randomized to receive one of two different doses of a piperacillin-tazobactam combination (8:1): a low dose (n = 23) of 50 and 6.25 mg of piperacillin and tazobactam per kg of body weight, respectively, or a high dose (n = 24) of 100 and 12.5 mg, respectively. The pharmacokinetic behavior of tazobactam was very similar to that observed for piperacillin, supporting the use of these two agents in a fixed-dose combination. No differences in the pharmacokinetics of piperacillin or tazobactam were observed between the two doses administered. The elimination parameters half-life and total body clearance decreased and increased, respectively, with increasing age, whereas volume parameters (volume of distribution and steady-state volume of distribution) remained relatively constant for both compounds. The primary metabolite of tazobactam, metabolite M1, was measurable in the plasma of 18 of the 47 study subjects; 17 of these 18 subjects received the high doses. More than 70% of the administered piperacillin and tazobactam doses were excreted unchanged in the urine over a 6-h collection period. These data combined with the known in vitro susceptibilities of a broad range of pediatric bacterial pathogens indicate that a dose of 100 mg of piperacillin and 12.5 of mg tazobactam per kg of body weight administered as a fixed-dose combination every 6 to 8 h would be appropriate to initiate clinical efficacy studies in infants and children for the treatment of systemic infections arising outside of the central nervous system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Goldstein F. W., Kitzis M. D. Susceptibility survey of piperacillin alone and in the presence of tazobactam. J Antimicrob Chemother. 1993 Jan;31 (Suppl A):23–28. doi: 10.1093/jac/31.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C. Comparative susceptibility profile of piperacillin/tazobactam against anaerobic bacteria. J Antimicrob Chemother. 1993 Jan;31 (Suppl A):29–38. doi: 10.1093/jac/31.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- Aronoff G. R., Sloan R. S., Brier M. E., Luft F. C. The effect of piperacillin dose on elimination kinetics in renal impairment. Eur J Clin Pharmacol. 1983;24(4):543–547. doi: 10.1007/BF00609901. [DOI] [PubMed] [Google Scholar]
- Batra V. K., Morrison J. A., Lasseter K. C., Joy V. A. Piperacillin kinetics. Clin Pharmacol Ther. 1979 Jul;26(1):41–53. doi: 10.1002/cpt197926141. [DOI] [PubMed] [Google Scholar]
- Bergan T., Williams J. D. Dose dependence of piperacillin pharmacokinetics. Chemotherapy. 1982;28(3):153–159. doi: 10.1159/000238070. [DOI] [PubMed] [Google Scholar]
- Bryson H. M., Brogden R. N. Piperacillin/tazobactam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1994 Mar;47(3):506–535. doi: 10.2165/00003495-199447030-00008. [DOI] [PubMed] [Google Scholar]
- Bush K., Macalintal C., Rasmussen B. A., Lee V. J., Yang Y. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother. 1993 Apr;37(4):851–858. doi: 10.1128/aac.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand J Infect Dis Suppl. 1990;74:235–248. [PubMed] [Google Scholar]
- Eklund A. E., Nord C. E. A randomized multicenter trial of piperacillin/tazobactam versus imipenem/cilastatin in the treatment of severe intra-abdominal infections. Swedish Study Group. J Antimicrob Chemother. 1993 Jan;31 (Suppl A):79–85. doi: 10.1093/jac/31.suppl_a.79. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Prior R. B. Comparative in vitro activities of piperacillin-tazobactam and ticarcillin-clavulanate. Antimicrob Agents Chemother. 1989 Aug;33(8):1268–1274. doi: 10.1128/aac.33.8.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Piperacillin, a new penicillin active against many bacteria resistant to other penicillins. Antimicrob Agents Chemother. 1978 Mar;13(3):358–367. doi: 10.1128/aac.13.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron J. A., Meyers B. R., Hirschman S. Z. Biliary concentrations of piperacillin in patients undergoing cholecystectomy. Antimicrob Agents Chemother. 1981 Feb;19(2):309–311. doi: 10.1128/aac.19.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashitani F., Hyodo A., Ishida N., Inoue M., Mitsuhashi S. Inhibition of beta-lactamases by tazobactam and in-vitro antibacterial activity of tazobactam combined with piperacillin. J Antimicrob Chemother. 1990 Apr;25(4):567–574. doi: 10.1093/jac/25.4.567. [DOI] [PubMed] [Google Scholar]
- Holmes B., Richards D. M., Brogden R. N., Heel R. C. Piperacillin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1984 Nov;28(5):375–425. doi: 10.2165/00003495-198428050-00002. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R., Aronoff S. C., Johenning S., Shlaes D. M., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with ampicillin and broad-spectrum penicillins against defined beta-lactamase-producing aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1986 Jun;29(6):980–985. doi: 10.1128/aac.29.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. A., Halstenson C. E., Kelloway J. S., Shapiro B. E., Zimmerman S. W., Tonelli A., Faulkner R., Dutta A., Haynes J., Greene D. S. Single-dose pharmacokinetics of piperacillin and tazobactam in patients with renal disease. Clin Pharmacol Ther. 1992 Jan;51(1):32–41. doi: 10.1038/clpt.1992.5. [DOI] [PubMed] [Google Scholar]
- Kearns G. L., Reed M. D. Clinical pharmacokinetics in infants and children. A reappraisal. Clin Pharmacokinet. 1989;17 (Suppl 1):29–67. doi: 10.2165/00003088-198900171-00005. [DOI] [PubMed] [Google Scholar]
- Kinzig M., Sörgel F., Brismar B., Nord C. E. Pharmacokinetics and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob Agents Chemother. 1992 Sep;36(9):1997–2004. doi: 10.1128/aac.36.9.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck N. A., Jacobus N. V., Petersen P. J., Weiss W. J., Testa R. T. Comparative in vitro and in vivo activities of piperacillin combined with the beta-lactamase inhibitors tazobactam, clavulanic acid, and sulbactam. Antimicrob Agents Chemother. 1989 Nov;33(11):1964–1969. doi: 10.1128/aac.33.11.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr Meeting the challenges of beta-lactamases. J Antimicrob Chemother. 1993 Jan;31 (Suppl A):1–8. doi: 10.1093/jac/31.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Mouton Y., Leroy O., Beuscart C., Chidiac C., Senneville E., Ajana F., Lecocq P. Efficacy, safety and tolerance of parenteral piperacillin/tazobactam in the treatment of patients with lower respiratory tract infections. J Antimicrob Chemother. 1993 Jan;31 (Suppl A):87–95. doi: 10.1093/jac/31.suppl_a.87. [DOI] [PubMed] [Google Scholar]
- Prince A. S., Neu H. C. New penicillins and their use in pediatrics. Pediatr Clin North Am. 1983 Feb;30(1):3–16. doi: 10.1016/s0031-3955(16)34315-2. [DOI] [PubMed] [Google Scholar]
- Reed M. D., Blumer J. L. Amoxicillin-potassium clavulanate: rational pharmacology meets clinical infectious disease. Clin Pharm. 1984 Nov-Dec;3(6):653–654. [PubMed] [Google Scholar]
- Reed M. D., Myers C. M., Yamashita T. S., Blumer J. L. Developmental pharmacology and therapeutics of piperacillin in gram-negative infections. Dev Pharmacol Ther. 1986;9(2):102–114. doi: 10.1159/000457082. [DOI] [PubMed] [Google Scholar]
- Reed M. D., Stern R. C., Bertino J. S., Jr, Myers C. M., Yamashita T. S., Blumer J. L. Dosing implications of rapid elimination of trimethoprim-sulfamethoxazole in patients with cystic fibrosis. J Pediatr. 1984 Feb;104(2):303–307. doi: 10.1016/s0022-3476(84)81019-7. [DOI] [PubMed] [Google Scholar]
- Reed M. D., Stern R. C., Myers C. M., Klinger J. D., Yamashita T. S., Blumer J. L. Therapeutic evaluation of piperacillin for acute pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 1987 Mar-Apr;3(2):101–109. doi: 10.1002/ppul.1950030212. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. beta-Lactamases of gram-negative bacteria: new challenges for new drugs. Clin Infect Dis. 1992 May;14(5):1089–1099. doi: 10.1093/clinids/14.5.1089. [DOI] [PubMed] [Google Scholar]
- Tan J. S., Wishnow R. M., Talan D. A., Duncanson F. P., Norden C. W. Treatment of hospitalized patients with complicated skin and skin structure infections: double-blind, randomized, multicenter study of piperacillin-tazobactam versus ticarcillin-clavulanate. The Piperacillin/Tazobactam Skin and Skin Structure Study Group. Antimicrob Agents Chemother. 1993 Aug;37(8):1580–1586. doi: 10.1128/aac.37.8.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumoorthi M. C., Asmar B. I., Buckley J. A., Bollinger R. O., Kauffman R. E., Dajani A. S. Pharmacokinetics of intravenously administered piperacillin in preadolescent children. J Pediatr. 1983 Jun;102(6):941–946. doi: 10.1016/s0022-3476(83)80030-4. [DOI] [PubMed] [Google Scholar]
- Tjandramaga T. B., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother. 1978 Dec;14(6):829–837. doi: 10.1128/aac.14.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Duchateau V., Lambert C., Husson M., Kinzig M., Sörgel F. Ex vivo pharmacodynamic study of piperacillin alone and in combination with tazobactam, compared with ticarcillin plus clavulanic acid. Antimicrob Agents Chemother. 1993 Sep;37(9):1860–1868. doi: 10.1128/aac.37.9.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Koup J. R., Opheim K. E., Adelman L. A., Levy J., Stull T. L., Clausen C., Smith A. L. Piperacillin pharmacokinetics in pediatric patients. Antimicrob Agents Chemother. 1982 Sep;22(3):442–447. doi: 10.1128/aac.22.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Logan M., Cooper M., Andrews J. M. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob Agents Chemother. 1991 Jun;35(6):1081–1084. doi: 10.1128/aac.35.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]