Abstract
Background
C-peptide increases forearm blood flow (FBF) in patients with Type 1 diabetes, probably by interaction with insulin, but not in healthy subjects. It is unclear if the vasodilating effect is sealed at normal fasting insulin concentrations.
Methods
The effects of C-peptide alone and during local hyperinsulinaemia were studied in healthy young men. Subjects received intra-arterial insulin at 6 pmol min−1 (low dose) or placebo for 60 min with subsequent coinfusion of C-peptide at increasing doses of 2–60 pmol min−1 in a double-blind crossover study (n = 8). In control experiments insulin at 30 pmol min−1 (high dose) was coinfused with C-peptide (n = 3). FBF was measured by strain-gauge plethysmography.
Results
Placebo had no effect on FBF (mean percentage change from baseline at 50 min −3.1%, 95% confidence interval [CI]−14.9, + 8.7). Insulin infusion slightly enhanced FBF by + 10.2% (95% CI −6.8, + 27.2; low dose) and + 17.6% (95% CI −38.8, + 74.0; high dose), respectively. The mean individual difference of the change in FBF between low-dose insulin and placebo was + 13.3% (95% CI −6.0, + 32.7; P = NS). Infusion of C-peptide increased local C-peptide concentrations from 1.8 ± 0.1 ng ml−1 to 6.1 ± 2.8 ng ml−1, but had no effect on FBF during placebo or hyperinsulinaemia (mean difference vs low dose insulin −16.0%, 95% CI −38.9, + 6.9).
Conclusion
The vasodilating effect of C-peptide seen in Type 1 diabetes is not detectable during fasting or hyperinsulinaemia in the forearm vasculature of healthy subjects. This suggests saturation of its vasodilating potency at insulin concentrations within the normal or in the supraphysiological range.
Keywords: C-peptide, forearm blood flow, insulin, vasodilation
Introduction
C-peptide has only trivial metabolic effects in humans [1–3] and it has been believed that the role of C-peptide is mainly to facilitate the folding of the proinsulin molecule. However, there is evidence that C-peptide can exert other physiological effects, as the administration of C-peptide decreased glomerular hyperfiltration in diabetic rats [4, 5], and augmented glucose utilization and improved autonomic nervous function in patients with Type 1 diabetes mellitus [6–8]. C-peptide has further been described to act as a vasodilator in patients with Type 1 diabetes mellitus, where short-term systemic infusions of C-peptide increased forearm blood flow (FBF) and brachial artery diameter at physiological concentrations [9]. Therefore, the lack of endogenous C-peptide in Type 1 diabetes has been proposed to contribute to the development of diabetic vascular complications [10, 11].
Interestingly, the vascular effects of C-peptide were found only in patients with Type 1 diabetes and not in healthy subjects [3, 9, 11], which is compatible with animal studies [12]. This is at variance with in vitro data showing that C-peptide acts as a potent concentration-dependent vasodilator in the presence of nondilating concentrations of insulin in isolated rat muscle arterioles from nondiabetic animals, whereas C-peptide alone is only a weak and variable vasodilator [13]. This suggests that C-peptide might require the presence of insulin for its vascular actions. However, it is unclear if the vasodilating potency is already saturated under normal conditions in humans or if hyperinsulinaemia could have an incremental effect on C-peptide-induced vasodilation.
In the present study the vasoactive potential of C-peptide on forearm resistance arteries was assessed under fasting conditions and during hyperinsulinaemia. Changes in FBF were studied using strain gauge plethysmography.
Materials and methods
The study was approved by the Ethics Committee of the University of Vienna and complies with the principles of the Declaration of Helsinki including current revisions and the GCP guidelines. Written informed consent was obtained from all subjects prior to enrolment.
Subjects
Thirteen healthy male volunteers between the ages of 19 and 34 years were included in this study. All subjects were nonsmokers and drug-free. In a complete health examination (including physical examination, ECG, laboratory screening and oral glucose tolerance test) within 14 days prior to the first study day no clinically relevant abnormalities were detected. Descriptive characteristics of the study population are summarized in Table 1. Subjects were studied after an overnight fast in a quiet room with an ambient temperature of 22°C.
Table 1.
Baseline physical characteristics
| Low-dose insulin group (n = 8) | High-dose insulin group (n = 3) | |
|---|---|---|
| Age (years) | 25 ± 1 | 29 ± 3 |
| Body mass index (kg m−2) | 23.5 ± 0.6 | 23.5 ± 1.1 |
| Systolic blood pressure (mmHg) | 129 ± 3 | 119 ± 3 |
| Diastolic blood pressure (mmHg) | 60 ± 2 | 58 ± 9 |
| Heart rate (min−1) | 66 ± 4 | 65 ± 5 |
Data are means ±SEM
Experimental protocol
The effect of mild hyperinsulinaemia on C-peptide-induced vasodilation was studied in eight subjects and followed a double-blind, placebo-controlled, crossover design with washout between the 2 trial days of at least 2 days.
On both study days a 27-G needle was inserted into the brachial artery of the nondominant arm for drug infusion and an intravenous cannula was inserted into the other arm for quantification of systemic insulin and C-peptide concentrations. After a 15-min resting period in supine position with saline infusion, allowing the participants to acclimatize to the needle, an intra-arterial infusion of insulin at 6 pmol min−1 (Huminsulin; Lilly, Fegersheim, France; mixed with heparinized blood to avoid adsorption to the syringe and to connecting tubing [14]) or placebo (0.9% saline) was started. After 60 min, increasing doses of C-peptide (Biotrend Chemikalien GmbH, Cologne, Germany) were coinfused intra-arterially. C-peptide doses were increased at 10-min intervals from 2 to 4, 8, 15, 30 and 60 pmol min−1, respectively [11].
Venous C-peptide and insulin concentrations were determined from the infusion arm in two additional subjects without measurement of FBF.
An open-label third study day was carried out in three subjects, where a higher local insulin dose of 30 pmol min−1 was infused [15]. The experiments were identical otherwise. Local C-peptide and insulin concentrations were determined in blood samples from a forearm vein of the intervention arm.
Systemic haemodynamics
Blood pressure was measured by an automated oscillometric device on the wrist of the control arm in 10-min intervals, pulse rate was monitored continuously by a finger pulse-oxymetric device (Hewlett Packard CMS patient monitor; Palo Alto, CA, USA) [16].
Forearm blood flow
FBF was measured by bilateral venous occlusion strain-gauge plethysmography [17, 18]. Briefly, inflatable upper arm cuffs and wrist cuffs were attached to both arms. Strain gauges were placed on the forearms and connected to plethysmographs (EC-6; D.E. Hokanson, Bellevue, WA, USA). Traces were analysed using the NIVP3 software (Version 5.25; Hokanson). Flow measurements were recorded for 9 s at 30-s intervals at baseline and during drug infusion, while the upper arm and wrist cuffs were inflated to 45 mmHg and 200 mmHg, respectively. FBF was expressed as the ratio of blood flow in the intervention to control arm and baseline ratio was defined as 100%. Local drug effects were expressed as percentage change over baseline obtained during saline infusion. For statistical analysis and graphical display one value of FBF was calculated for every 10-min interval as the mean of the ten measurements obtained during the final 5 min of the respective time period.
Laboratory parameters
Every 10 min, venous blood samples were drawn from the non-infused arm to determine glucose plasma concentrations (Glucose Analyser; Beckman Inc., Fullerton, CA, USA). Laboratory analyses for insulin and C-peptide concentrations were carried out according to standard procedures at the Institute of Medical and Chemical Laboratory Diagnostics, Allgemeines Krankenhaus Wien.
Statistical analysis
All data sets were tested for normal distribution using the Kolmogorov–Smirnov test. Due to lognormal distribution, data were log-transformed before anova. Percent changes of FBF from baseline were used for statistical analysis of the effects of insulin over time and vs placebo control. For statistical comparison of the effects of C-peptide with insulin or placebo, blood flow recorded before coinfusion experiments was taken as new baseline and mean individual difference between treatment groups calculated. All calculations were performed using the Statistica software package (Release 5.1; StatSoft Inc., Tulsa, OK, USA). P ≤ 0.05 was considered significant. Values are expressed as means ± SEM unless indicated otherwise.
Results
The drugs under study were well-tolerated and no systemic side-effects or local complications were reported.
Systemic haemodynamics
No significant differences in blood pressure or pulse rate were observed at baseline or between the 2 study days, and the drugs under study had no effect on systemic haemodynamics (data not shown).
Forearm blood flow
FBF at baseline was 4.1 ml min−1 100 ml−1 (95% confidence interval [CI] 2.5, 5.6) and 5.2 ml min−1 100 ml−1 (95% CI 2.8, 7.7) on study days with placebo and low-dose insulin (P = NS between study days).
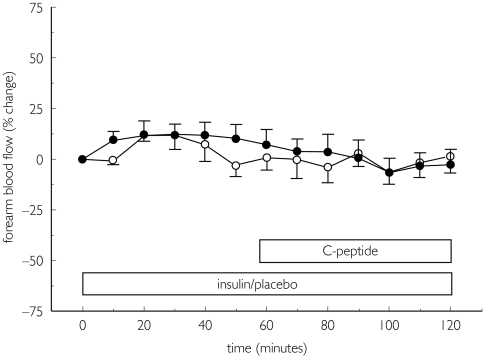
Placebo had no effect on FBF (mean percentage change from baseline at 50 min −3.1%, 95% CI −14.9, + 8.7). Insulin had only a small effect on FBF and caused an increase of + 10.2% (95% CI −6.8, + 27.2) at a dose of 6 pmol min−1 (P = NS vs baseline and placebo; Fig. 1). The mean individual difference of the change in FBF between insulin and placebo days was + 13.3% (95% CI −6.0, + 32.7; P = NS).
Figure 1.
Forearm blood flow during intra-arterial coinfusion of insulin at 6 pmol min−1 (•) or placebo ( ) with cumulative doses of C-peptide (2, 4, 8, 15, 30, 60 pmol min−1, infusion period 10 min each). Data are expressed as percentage change from baseline as means ± SEM, n = 8.
) with cumulative doses of C-peptide (2, 4, 8, 15, 30, 60 pmol min−1, infusion period 10 min each). Data are expressed as percentage change from baseline as means ± SEM, n = 8.
Coinfusion of C-peptide changed FBF by + 3.0% (95% CI −15.3, + 21.4) and −12.9% (95% CI −30.6, + 4.8) from preceding placebo or low-dose insulin administration, respectively (Fig. 1). The mean individual difference between treatment groups was −16.0% (95% CI −38.9, + 6.9).
High-dose insulin infusion increased FBF by + 17.6% (95% CI −38.8, + 74.0) from baseline. Coinfusion of C-peptide had no effect on FBF (mean change + 0.1%, 95% CI −15.2, + 15.5; P = Ns, n = 3).
Laboratory parameters
Glucose levels remained in the physiological range throughout the study and no substitution was necessary. Venous blood glucose tended to decrease over time, from a mean of 85 ± 3 mg dl−1 to 80 ± 1 mg dl−1 during placebo and from 86 ± 4 mg dl−1 to 78 ± 3 mg dl−1 during local insulin infusion (P = NS). High-dose insulin also had no significant effect on plasma glucose levels. Co-administration of C-peptide had no effect on venous glucose levels.
Insulin concentrations in the intervention arm increased more than three-fold at 120 min from a baseline of 8.4 ± 1.2 to 28.5 ± 9.5 µU ml−1 (n = 2) during infusion of insulin at 6 pmol min−1 and increased nearly ten-fold from a baseline of 12.6 ± 3.6 to 115.7 ± 32.3 µU ml−1 (n = 3) during insulin at 30 pmol min−1. Systemic insulin concentrations slightly decreased from baseline values of 8.0 ± 0.8 to 6.8 ± 0.8 µU ml−1 during low-dose insulin infusion (n = 8, P = NS) and from 14.4 ± 4.9 at baseline to 10.0 ± 1.6 µU ml−1 during high-dose insulin infusion (n = 3, P = NS).
Local C-peptide concentrations were comparable between study days. Local forearm C-peptide was 1.8 ± 0.1 ng ml−1 and increased to 6.1 ± 2.8 ng ml−1 after infusion of C-peptide (n = 2). Systemic C-peptide concentrations remained unchanged (data not shown).
Discussion
In the present experiments C-peptide exerted no direct or insulin-enhancing effect on FBF in healthy humans, despite significantly increased local concentrations of the drugs under study.
It is well known that insulin causes a gradual vasodilating effect after intra-arterial infusion into the forearm [19]. In our study we selected a dose to increase local concentrations of insulin without altering basal blood flow. Despite tripled local insulin concentrations, only little forearm vasodilation was noted, which is in agreement with previous other studies [15]. However, substantially higher local forearm concentrations of insulin of approximately 115 µU ml−1 in venous outflow also had only little impact on regional blood flow. The latter control experiments were conducted in a small number of subjects and the lack of significant increase in blood flow could be due to limited power, given the variability of effects in healthy subjects [20]. Also, minor differences in body mass index and metabolic state may influence outcome in a small sample size. In particular, insulin sensitivity can affect vascular responses to exogenous insulin infusions [21]. Further, the vascular effect of insulin in the forearm depends on adequate glucose availability, as demonstrated in coinfusion experiments employing d-glucose [22]. Systemic haemodynamics were not affected by local infusion of insulin, which also had no effect on circulating insulin concentrations.
Our results with C-peptide infusion differ from findings in patients with Type 1 diabetes, where C-peptide alone acted as a vasodilator at equivalent plasma concentrations [6–9, 23]. However, the absence of vascular effects of C-peptide in healthy animals [12, 13] and in healthy humans [3, 9, 11] has been consistently reported in the literature. The lack of vascular effects of C-peptide in healthy subjects could reflect pharmacodynamic saturation [24], which does not contradict that C-peptide acts as a vasodilator in patients with Type 1 diabetes deprived of C-peptide. This condition may eventually lead to up-regulation of the C-peptide signal transduction pathway. Although no specific receptor for C-peptide has been described yet and nonreceptor membrane interactions have been suggested to explain the mechanism [25], recent binding studies have shown a typical receptor interaction pattern [24, 26]. C-peptide stimulates Na+-K+-ATPase activity in rat renal tubular cells [27] and restores erythrocyte deformability in patients with Type 1 diabetes through the same mechanism [28].
The combination of insulin with C-peptide increased vasodilation in in vitro studies [13] and in subjects with Type 1 diabetes mellitus [4–8]. Previous studies addressing the role of C-peptide in healthy subjects were limited by different aspects. First, no exogenous insulin was added in most studies [9, 11], which only investigated effects at ambient insulin concentrations. Second, when insulin was administered with C-peptide intravenously, systemic effects and metabolic changes could have influenced the vascular responsiveness or altered basal tone. This has to be considered, since insulin's systemic vascular action is at least partly linked to its metabolic effects [29]. Also, nervous system activation [30, 31] with release of vasoactive hormones can occur. We therefore studied the functional effects of C-peptide during local hyperinsulinaemia in healthy humans.
In conclusion, intra-arterial infusions of C-peptide with or without insulin did not cause significant forearm vasodilation in healthy humans. This suggests that patients with Type 1 diabetes mellitus are more susceptible to the vascular effects of C-peptide than healthy subjects, where pharmacodynamic saturation might already be reached.
References
- 1.Hagen C. Studies on the subunits of the human glycoprotein hormones in relation to reproduction. Scand J Clin Lab Invest Suppl. 1977;148:1–19. doi: 10.3109/00365517809091510. [DOI] [PubMed] [Google Scholar]
- 2.Hoogwerf BJ, Bantle JP, Gaenslen HE, et al. Infusion of synthetic human C-peptide does not affect plasma glucose, serum insulin, or plasma glucagons in healthy subjects. Metabolism. 1986;35:122–125. doi: 10.1016/0026-0495(86)90111-3. [DOI] [PubMed] [Google Scholar]
- 3.Wahren J, Ekberg K, Johansson J, et al. Role of C-peptide in human physiology. Am J Physiol Endocrinol Metab. 2000;278:E759–E768. doi: 10.1152/ajpendo.2000.278.5.E759. [DOI] [PubMed] [Google Scholar]
- 4.Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose–response study. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:67–73. doi: 10.1007/s00210-001-0502-1. [DOI] [PubMed] [Google Scholar]
- 5.Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int. 2001;60:1258–1265. doi: 10.1046/j.1523-1755.2001.00964.x. [DOI] [PubMed] [Google Scholar]
- 6.Forst T, Kunt T, Pfutzner A, Beyer J, Wahren J. New aspects on biological activity of C-peptide in IDDM patients. Exp Clin Endocrinol Diabetes. 1998;106:270–276. doi: 10.1055/s-0029-1212190. [DOI] [PubMed] [Google Scholar]
- 7.Johansson BL, Kernell A, Sjoberg S, Wahren J. Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab. 1993;77:976–981. doi: 10.1210/jcem.77.4.8408474. [DOI] [PubMed] [Google Scholar]
- 8.Johansson BL, Borg K, Fernqvist-Forbes E, Odergren T, Remahl S, Wahren J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia. 1996;39:687–695. doi: 10.1007/BF00418540. [DOI] [PubMed] [Google Scholar]
- 9.Fernqvist-Forbes E, Johansson BL, Eriksson MJ. Effects of C-peptide on forearm blood flow and brachial artery dilation in patients with type I diabetes mellitus. Acta Physiol Scand. 2001;172:159–165. doi: 10.1046/j.1365-201x.2001.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Wahren J, Johansson BL, Wallberg-Henriksson H. Does C-peptide have a physiological role? Diabetologia. 1994;37(Suppl 2):S99–S107. doi: 10.1007/BF00400832. [DOI] [PubMed] [Google Scholar]
- 11.Johansson BL, Linde B, Wahren J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type I (insulin-dependent) diabetic patients. Diabetologia. 1992;35:1151–1158. doi: 10.1007/BF00401369. [DOI] [PubMed] [Google Scholar]
- 12.Jansson L. Effects of C-peptide on splanchnic blood flow in anaesthetised rats. Med Sci Res. 1995;23:157–158. [Google Scholar]
- 13.Jensen ME, Messina EJ. C-peptide induces a concentration-dependent dilation of skeletal muscle arterioles only in the presence of insulin. Am J Physiol. 1999;276:H1223–H1228. doi: 10.1152/ajpheart.1999.276.4.H1223. [DOI] [PubMed] [Google Scholar]
- 14.Kerchner J, Colaluca DM, Juhl RP. Effect of whole blood on insulin adsorption onto intravenous infusion systems. Am J Hosp Pharm. 1980;37:1323–1325. [PubMed] [Google Scholar]
- 15.Ueda S, Petrie JR, Cleland SJ, Elliott HL, Connel JM. The vasodilating effect of insulin is dependent on local glucose uptake: a double blind placebo-controlled study. J Clin Endocrinol Metab. 1998;83:2126–2131. doi: 10.1210/jcem.83.6.4897. [DOI] [PubMed] [Google Scholar]
- 16.Wolzt M, Schmetterer L, Rheinberger A, et al. Comparison of non-invasive methods for the assessment of haemodynamic drug effects in healthy male and female volunteers: sex differences in cardiovascular responsiveness. Br J Clin Pharmacol. 1995;39:347–359. doi: 10.1111/j.1365-2125.1995.tb04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hokanson DE, Sumner DS, Strandness DE., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22:25–29. doi: 10.1109/tbme.1975.324535. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 19.Tack CJ, Schefman AE, Willems JL, Thien T, Lutterman JA, Smits P. Direct vasodilator effects of physiological hyperinsulinaemia in human skeletal muscle. Eur J Clin Invest. 1996;26:772–778. doi: 10.1046/j.1365-2362.1996.2020551.x. [DOI] [PubMed] [Google Scholar]
- 20.Lind L, Fugmann A, Millgard J, Berne C, Lithell H. Insulin-mediated vasodilatation, but not glucose uptake or endothelium-mediated vasodilatation, is enhanced in young females compared with males. Clin Sci. 2002;102:241–246. [PubMed] [Google Scholar]
- 21.Fossum E, Hoieggen A, Moan A, Rostrup M, Nordby G, Kjeldsen SE. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension. 1998;32:838–843. doi: 10.1161/01.hyp.32.5.838. [DOI] [PubMed] [Google Scholar]
- 22.Cleland SJ, Petrie JR, Ueda S, Elliott H, Connell J. Insulin-mediated vasodilation and glucose uptake are functionally linked in humans. Hypertension. 1999;33:554–558. doi: 10.1161/01.hyp.33.1.554. [DOI] [PubMed] [Google Scholar]
- 23.Johansson BL, Pernow J. C-peptide potentiates the vasoconstrictor effect of neuropeptide Y in insulin-dependent diabetic patients. Acta Physiol Scand. 1999;165:39–44. doi: 10.1046/j.1365-201x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- 24.Rigler R, Pramanik A, Jonasson P, et al. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci USA. 1999;96:13318–13323. doi: 10.1073/pnas.96.23.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ido Y, Vindigni A, Chang K, et al. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science. 1997;277:563–566. doi: 10.1126/science.277.5325.563. [DOI] [PubMed] [Google Scholar]
- 26.Johansson J, Ekberg K, Shafqat J, et al. Molecular effects of proinsulin C-peptide. Biochem Biophys Res Commun. 2002;295:1035–1040. doi: 10.1016/s0006-291x(02)00721-0. [DOI] [PubMed] [Google Scholar]
- 27.Ohtomo Y, Aperia A, Sahlgren B, Johansson BL, Wahren J. C-peptide stimulates rat renal tubular Na+, K(+)-ATPase activity in synergism with neuropeptide Y. Diabetologia. 1996;39:199–205. doi: 10.1007/BF00403963. [DOI] [PubMed] [Google Scholar]
- 28.Kunt T, Schneider S, Pfutzner A, et al. The effect of human proinsulin C-peptide on erythrocyte deformability in patients with Type I diabetes mellitus. Diabetologia. 1999;42:465–471. doi: 10.1007/s001250051180. [DOI] [PubMed] [Google Scholar]
- 29.Cardillo C, Kilocyne CM, Nambi SS, Cannon RO, Quon MJ, Panza JA. Vasodilator response to systemic but not to local hyperinsulinemia in the human forearm. Hypertension. 1998;32:740–745. doi: 10.1161/01.hyp.32.4.740. [DOI] [PubMed] [Google Scholar]
- 30.Hausberg M, Hoffman RP, Somers VK, Sinkey CA, Mark AL, Anderson EA. Contrasting autonomic and hemodynamic effects of insulin in healthy elderly versus young subjects. Hypertension. 1997;29:700–705. doi: 10.1161/01.hyp.29.3.700. [DOI] [PubMed] [Google Scholar]
- 31.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]