Abstract
Objective To review the evidence for the use of bisphosphonates to reduce skeletal morbidity in cancer patients with bone metastases.
Data sources Electronic databases, scanning reference lists, and consultation with experts and pharmaceutical companies. Foreign language papers were included.
Study selection Included trials were randomised controlled trials of patients with malignant disease and bone metastases who were treated with oral or intravenous bisphosphonate compared with another bisphosphonate, placebo, or standard care. All trials measured at least one outcome of skeletal morbidity.
Results 95 articles were identified; 30 studies fulfilled inclusion criteria. In studies that lasted ≥ 6 months, compared with placebo bisphosphonates significantly reduced the odds ratio for fractures (vertebral 0.69, 95% confidence interval 0.57 to 0.84, P < 0.0001; non-vertebral 0.65, 0.54 to 0.79, P < 0.0001; combined 0.65, 0.55 to 0.78, P < 0.0001), radiotherapy (0.67, 0.57 to 0.79, P < 0.0001), and hypercalcaemia (0.54, 0.36 to 0.81, P = 0.003) but not for orthopaedic surgery (0.70, 0.46 to 1.05, P = 0.086) or spinal cord compression (0.71, 0.47 to 1.08, P = 0.113). The reduction in orthopaedic surgery was significant in studies that lasted over a year (0.59, 0.39 to 0.88, P = 0.009). Use of bisphosphonates significantly increased time to first skeletal related event but did not increase survival. Subanalyses showed that most evidence supports use of intravenous aminobisphosphonates.
Conclusions In people with metastatic bone disease bisphosphonates significantly decrease skeletal morbidity, except for spinal cord compression and increased time to first skeletal related event. Treatment should start when bone metastases are diagnosed and continue until it is no longer clinically relevant.
Introduction
Metastatic bone disease causes substantial morbidity among cancer patients. Complications from bone metastases include pathological fracture, hypercalcaemia, nerve root compression, spinal cord compression, bone marrow infiltration, pain, and reduced mobility.1,2
Although there are many therapeutic options for the treatment of such complications, none is completely satisfactory, even when used in combination. These patients continue to represent a major therapeutic challenge for the clinician.
Bisphosphonates work by several different mechanisms to reduce both bone resorption and bone formation.3,4 In vitro work has shown that bisphosphonates may have a direct action on tumour cells—for example, by inducing apoptosis, inhibiting matrix metalloproteinase-1, and inhibiting adhesion of tumour cells within the bone.5 Bisphosphonates can be divided into two groups. Those resembling pyrophosphate (for example, clodronate, etidronate) act as analogues of ATP and inhibit ATP dependent intracellular enzymes. The second group—aminobisphosphonates (for example, pamidronate, zoledronic acid)—inhibit enzymes of the mevalonate pathway, disrupting the signalling functions of key regulatory proteins.6–9 The net effect in both groups is inhibition of osteoclast function, which leads to a decrease in bone resorption.
We have assessed the evidence for the role of bisphosphonates in the reduction of skeletal morbidity in patients with bone metastases. We looked at who would benefit from treatment, when treatment should be started, and for how long it should be continued. We also compared different drugs, routes of administration, and tolerability.
Methods
Inclusion criteria
We included randomised controlled trials of patients with proved malignant disease and bone metastases. All trials measured at least one skeletal morbidity outcome. We included patients with multiple myeloma but excluded other haematological malignancies. Intervention could be either oral or intravenous bisphosphonate in the experimental group compared with another bisphosphonate, placebo, or standard care.
Outcome measures
Our primary outcome measures were time to first skeletal related event and reduction in skeletal morbidity assessed by pathological fractures (vertebral, non-vertebral, combined), radiotherapy to bone metastases, spinal cord compression, orthopaedic surgery, and hypercalcaemia. We did not include pain relief as an end point because another systematic review analysing this effect has recently been published.10 Secondary analyses determined the efficacy of bisphosphonates over time, the efficacy of one bisphosphonate over another, and the effect in different disease groups, specifically breast cancer and multiple myeloma, and compared routes of administration.
Identification of studies
We identified studies by searching electronic databases, scanning reference lists of articles, and consulting experts in the specialty (United Kingdom and abroad) and drug companies for unpublished data. The search strategy included cancer terms, bisphosphonates (cas numbers, generic, chemical and trade names (UK and foreign), and a recognised filter for identifying randomised controlled trials.11 No limit was applied to language; nine foreign papers were translated from Russian, Chinese, and European languages. This search was applied to Medline (1966-present) and CancerLit (1975-present) and adapted for Embase (1980-present), Science Citation Index Expanded (1981-present), and pre-Medline electronic databases. We also reviewed Cochrane databases and the database of abstracts of reviews of effectiveness (DARE). (The full electronic search strategy is available from authors). The last search was run on 19 June 2001. In addition, we hand searched Journal of Clinical Oncology 2001, European Journal of Cancer 2001, and Bone 2001 and meeting abstracts (1999-2001).
We reviewed the titles and abstracts of articles identified by the search strategy and identified possible articles. If no electronic abstract was available, or it was unclear, we obtained full text articles. Studies were then assessed by two independent reviewers using inclusion-exclusion sheets and data extraction forms developed for this review.
All randomised controlled trials were assessed and graded for allocation concealment according to Cochrane guidelines12 (A-adequate; B-unclear; C-inadequate; D-not used). Blinding of studies was recorded as open, single blind, or double blind. All studies were included at this stage, irrespective of blinding or allocation concealment.
Data synthesis
For most studies, we extracted outcome data in dichotomous form as proportions and used χ2 tests to compare groups. All analyses were performed with StatXact.13 For studies combined in a meta-analysis, we used odds ratios as a summary measure for each outcome. Studies were weighted with the inverse variance method. As we expected clinical heterogeneity to exist between studies we decided a priori that a random effects model would be applied to all meta-analyses.
Results
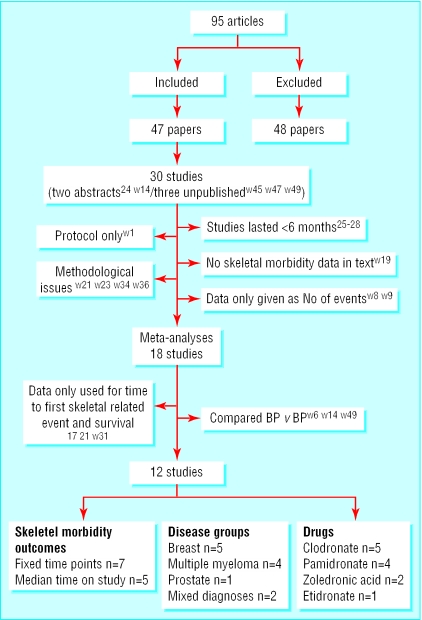
Forty seven papers describing 30 studies fulfilled the inclusion criteria for this review (fig 1). Data extracted from 18 studies were eligible for inclusion in the meta-analyses. The meta-analysis also included three large trials of zoledronic acid, which were unpublished at the time of inclusion in this review. We obtained full written permission to use these data. We are aware of further unpublished trials of ibandronic acid but were not able to include the data in this review. Details of all included (table A) and excluded (table B) trials plus a complete list of references (w1-w98) can be found on bmj.com.
Fig 1.
Flow diagram of studies (see table A on bmj.com for full details of the 47 included studies and table B on bmj.com for details of the 48 excluded studies)
For three of the 18 studies, data could be used only for time to first skeletal related event. Of the remaining studies, three compared two bisphosphonates and 12 compared a bisphosphonate with placebo or control.
Primary end points
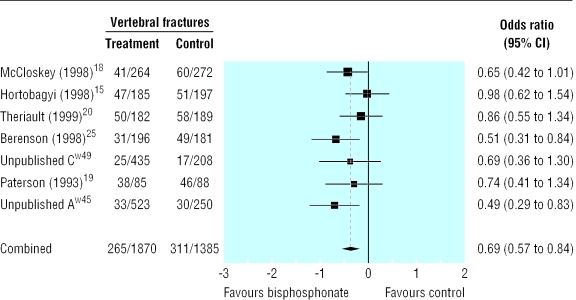
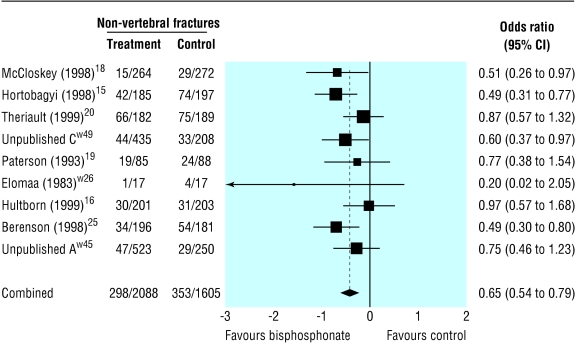
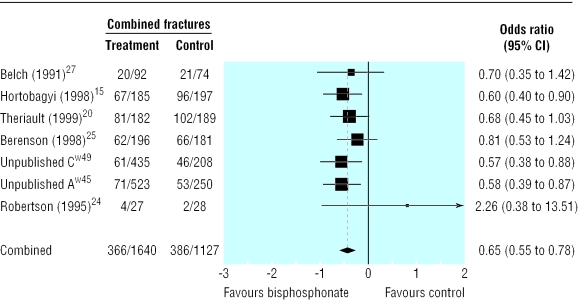
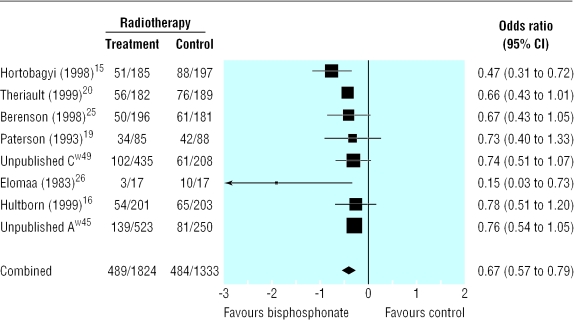
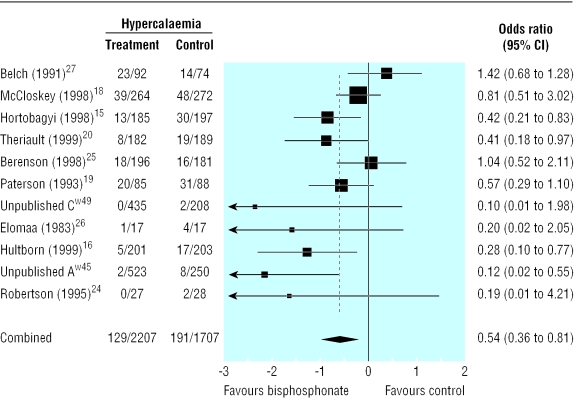
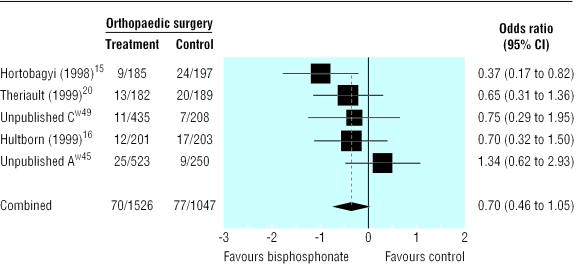
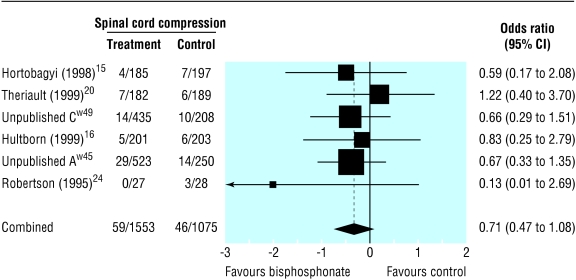
Compared with placebo, bisphosphonates significantly reduced the odds ratio for vertebral fractures (0.69, 95% 0.57 to 0.84, fig 2), non-vertebral fractures (0.65, 0.54 to 0.79, fig 3), combined fractures (0.65, 0.55 to 0.78, fig 4), radiotherapy (0.67, 0.57 to 0.79, fig 5), and hypercalcaemia (0.54, 0.36 to 0.81, fig 6) but not orthopaedic surgery (0.70, 0.46 to 1.05, fig 7) or spinal cord compression (0.71, 0.47 to 1.08, P = 0.113, fig 8). The risk of an individual skeletal related event for those taking bisphosphonates was 65% of the risk in patients not taking bisphosphonates for non-vertebral fractures, 69% for vertebral fractures, 67% for radiotherapy, and 54% for hypercalcaemia.
Fig 2.
Forest plot for vertebral fractures (3238 patients)
Fig 3.
Forest plot for non-vertebral fractures (3376 patients)
Fig 4.
Forest plot for combined fractures (2587 patients)
Fig 5.
Forest plot for radiotherapy (3140 patients)
Fig 6.
Forest plot for hypercalcaemia (3894 patients)
Fig 7.
Forest plot for orthopaedic surgery (2556 patients)
Fig 8.
Forest plot for spinal cord compression (2628 patients)
Ten studies included in the analyses recorded time to first skeletal related event for patients treated with bisphosphonate versus control (including two unpublished studies (A and C), R Murphy, Novartis, personal communication).14–21 We could not statistically combine data from different studies. Eight studies showed a significant increase in time to first event for patients who received bisphosphonate: four used intravenous pamidronate,14–16,20 two used intravenous zoledronic acid (studies A and C, R Murphy, Novartis, personal communication), and two used oral clodronate.17,21 In contrast two studies that used oral clodronate18,19 did not show a significant difference in time to first event. One study that compared zoledronic acid with pamidronate showed no difference in time to first event between the two drugs (study B, R Murphy, Novartis, personal communication).
Secondary end points
Time—Table 1 shows the results of analysis of individual skeletal morbidity by time. Reduction in the need for radiotherapy was significant at six months, episodes of hypercalcaemia at six months, and non-vertebral fractures at 12 months. The need for orthopaedic surgery progressively decreased with narrowing of the confidence interval over time, reaching significance at 24 months. Studies of less than six months' duration did not show significant results for any skeletal morbidity outcome.22–25
Table 1.
Summary statistics from pooled analysis at fixed time points for effect of bisphosphonates on radiotherapy, non-vertebral fractures, orthopaedic surgery, and hypercalcaemia in patients with metastatic bone disease
| Months of treatment | No of studies | No of patients | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Radiotherapy | ||||
| 6-11 | 4 | 1903 | 0.60 (0.47 to 0.77) | 0.0001 |
| 12-17 | 5 | 1807 | 0.54 (0.38 to 0.76) | 0.0001 |
| 18-23 | 3 | 1130 | 0.58 (0.44 to 0.76) | 0.0001 |
| ≥24 | 2 | 753 | 0.56 (0.40 to 0.78) | 0.001 |
| Non-vertebral fractures | ||||
| 6-11 | 4 | 1903 | 0.75 (0.50 to 1.14) | 0.179 |
| 12-17 | 4 | 1430 | 0.68 (0.48 to 0.96) | 0.031 |
| 18-23 | 2 | 753 | 0.68 (0.41 to 1.12) | 0.129 |
| ≥24 | 2 | 753 | 0.65 (0.37 to 1.14) | 0.132 |
| Orthopaedic surgery | ||||
| 6-11 | 3 | 1526 | 0.92 (0.36 to 2.35) | 0.866 |
| 12-17 | 3 | 1396 | 0.61 (0.37 to 1.01) | 0.054 |
| 18-23 | 2 | 753 | 0.52 (0.26 to 1.05) | 0.067 |
| ≥24 | 2 | 753 | 0.49 (0.28 to 0.86) | 0.013 |
| Hypercalcaemia | ||||
| 6-11 | 5 | 1916 | 0.42 (0.24 to 0.74) | 0.003 |
| 12-17 | 5 | 1807 | 0.50 (0.28 to 0.90) | 0.02 |
| 18-23 | 3 | 1130 | 0.56 (0.27 to 1.17) | 0.12 |
| ≥24 | 2 | 753 | 0.42 (0.34 to 0.51) | 0.0001 |
Disease groups—In the five trials among patients with breast cancer, compared with placebo bisphosphonates significantly reduced the odds ratio for non-vertebral fractures (0.80, 95% confidence interval 0.64 to 0.99), combined fractures (0.75, 0.61 to 0.93), radiotherapy (0.65, 0.54 to 0.79), orthopaedic surgery (0.59, 0.43 to 0.83), and hypercalcaemia (0.43,0.29 to 0.63) but not spinal cord compression (0.87, 0.44 to 1.73) or vertebral fractures (0.87, 0.71 to 1.06). By contrast, in the three trials among patients with multiple myeloma, the odds ratios were significantly reduced for vertebral fractures (0.58, 0.42 to 0.81) but not for hypercalcaemia (0.97, 0.69 to 1.37). In one trial that studied patients with prostate cancer the odds ratio was significantly reduced for combined fractures (0.57, 0.38 to 0.89) (study C, R Murphy, Novartis, personal communication). Two trials included patients with various cancer diagnoses (study A, R Murphy, Novartis, personal communication).26
Route of administration—Six trials studied intravenous bisphosphonates (pamidronate, zoledronic acid) versus placebo or control (studies (A and C), R Murphy, Novartis, personal communication).15,16,20,27 Results were similar to those from the primary analysis (table 2). Treatment with oral bisphosphonates (five trials; four clodronate,18,19,26,28 one etidronate29) significantly reduced the odds ratio for vertebral and non-vertebral fractures (table 2). The reduction in radiotherapy was not significant, but only 193 patients contributed to this analysis. The reduction in hypercalcaemia was also not significant, but this analysis was weighted by one study in patients with myeloma,18 which contributed over half of the 1064 patients.
Table 2.
Meta-analyses of comparison of effect of intravenous versus oral bisphosphonates on skeletal morbidity in patients with metastatic bone disease
|
Intravenous
|
Oral
|
|||||
|---|---|---|---|---|---|---|
| Outcome | No of patients | Odds ratio (95% CI) | P value | No of patients | Odds ratio (95% CI) | P value |
| Vertebral fractures | 2543 | 0.69 (0.52 to 0.91) | 0.009 | 695 | 0.68 (0.48 to 0.97) | 0.032 |
| Non-vertebral fractures | 2947 | 0.78 (0.63 to 0.96) | 0.018 | 729 | 0.59 (0.37 to 0.93) | 0.024 |
| Combined fractures | 2543 | 0.64 (0.53 to 0.77) | 0.0001 | 632 | 0.93 (0.35 to 2.50) | 0.89 |
| Radiotherapy | 2947 | 0.66 (0.56 to 0.78) | 0.0001 | 193 | 0.39 (0.09 to 1.79) | 0.228 |
| Orthopaedic surgery | 2570 | 0.70 (0.46 to 1.05) | 0.086 | — | — | — |
| Hypercalcaemia | 2930 | 0.40 (0.22 to 0.73) | 0.003 | 1064 | 0.78 (0.51 to 1.20) | 0.263 |
Individual bisphosphonate—Pamidronate significantly reduced all skeletal morbidity end points except spinal cord compression. Zoledronic acid studies showed similar results but were shorter and therefore the reduction in orthopaedic surgery did not reach significance (0.66, 0.39 to 1.14, P = 0.135). One trial directly compared pamidronate with zoledronic acid and did not show any difference between the two drugs (study C, R Murphy, Novartis, personal communication). Trials of clodronate were smaller, and only the results for vertebral and non-vertebral fractures reached significance.
Survival and toxicity —None of the individual studies found a significant difference in survival between patients treated with bisphosphonates and controls. Few data could be extracted from papers regarding performance status or quality of life because of inconsistencies in both the populations studied and the use of outcome measures. All bisphosphonates were well tolerated. Generally oral medications were associated with increased incidence of gastrointestinal side effects, but these were often reflected in placebo groups. Aminobisphosphonates were associated with a higher proportion of acute phase reactions.
Discussion
This systematic review supports the use of bisphosphonates to reduce skeletal morbidity in cancer patients with metastatic bone disease. Though some evidence based guidelines have been developed,30,31 clinical practice varies between centres. We have provided some answers relating to the clinical use of bisphosphonates.
Interpretation from available evidence—primary analyses
Bisphosphonates reduce skeletal morbidity in cancer patients with metastatic bone disease. The primary analyses show a highly significant reduction in fractures, need for radiotherapy, and hypercalcaemia in patients receiving bisphosphonates. The reduction in need for orthopaedic surgery becomes significant with time, the odds ratio being 0.587 (0.393 to 0.875, P < 0.009) for studies that lasted at least 12 months. There was no reduction in the incidence of spinal cord compression. This is a rare event compared with other skeletal morbidity end points, and studies were insufficiently powered to show a difference between bisphosphonates and control. Additionally spinal cord compression can be caused by non-osseous metastases.
As we used an extensive search strategy and broad inclusion criteria, we believe this study is as complete as possible. Though unpublished work has not been peer reviewed, included studies were critically assessed and met Cochrane criteria for robust methodology. We are aware of unpublished work that we were unable to include, which may result in publication bias. Any future updates would include data once it has become available in the public domain.
We were unable to include results of all of the studies in the meta-analyses because of differences in the way results were reported. Our attempts to contact all authors for raw data were rarely successful. Sensitivity analyses were not performed as this would have resulted in inclusion of recent larger studies and exclusion of older studies performed when the reporting of methodology was less robust, which may have introducing an element of bias.
Interpretation from available evidence—secondary analyses
Most of the trials of bisphosphonates have been performed in patients with breast cancer and multiple myeloma. The increased reduction in vertebral fractures in patients with myeloma compared with patients with breast cancer may be explained by more active disease in the vertebrae in myeloma patients. Lack of reduction in hypercalcaemia in patients with myeloma was surprising but may reflect mechanisms other than action of parathyroid hormone related protein and cytokines32; in particular renal handling of calcium may be impaired.32,33 Two recently released Cochrane reviews have looked at these disease groups, and their results are consistent with our findings.34,35
Regarding other disease groups, one study in patients with prostate cancer showed a significant reduction in combined fractures and a trend towards a reduction in radiotherapy (study C, R Murphy, Novartis, personal communication). This study did not last long enough to show a reduction in orthopaedic surgery. Preliminary results from another study in patients with prostate cancer also indicated that treatment delays the development of skeletal morbidity.36 The case is less clear for patients with other solid tumours, particularly those that have shorter prognoses (less than six months). Some of the trials in the primary analysis included patients with various tumours (study A, R Murphy, Novartis, personal communication)26 but individual data could not be extracted.
Most patients in clinical trials had osteolytic bone metastases on imaging studies. Recent guidelines from the American Society for Clinical Oncology (ASCO) for management of breast cancer recommend the use of bisphosphonates if there is lytic bone destruction.30 They note that there is insufficient evidence to recommend bisphosphonates for asymptomatic patients with an abnormal result on a bone scan. However, our results suggest that bisphosphonates should be started at diagnosis of bone metastases as time to first skeletal related event can be significantly delayed. This delay is likely to result in cost savings for the NHS.37 While it is also probable that patients' quality of life will be improved by early treatment and subsequent delay in time to skeletal morbidity, there are few data on quality of life from existing trials to support this premise.
What is already known on this topic
Individual studies have shown that patients with breast cancer who receive bisphosphonates to treat skeletal morbidity may experience a benefit
The magnitude of benefit, who to treat, when to treat, and for how long remains unclear
What this study adds
For patients with cancer and bone metastases, pooled results show that treatment with bisphosphonates is associated with a significant reduction in all skeletal morbidity end points except spinal cord compression
Bisphosphonates significantly increase the time to first skeletal related event, suggesting they should be started when bone metastases are diagnosed
Bisphosphonates reduce skeletal morbidity and should be continued until no longer clinically relevant; they do not affect survival
Most evidence supports the use of intravenous aminobisphosphonates, but further studies are needed to determine best drug and route
Clinical inferences
Our findings show that bisphosphonates need to be given for at least six months before an effect is seen on skeletal morbidity outcomes. Studies that lasted less than six months did not show a reduction in skeletal related events,22–25 although this may reflect the small numbers and low event rates in these studies. In addition, a reduction in orthopaedic surgery was not seen until 12-24 months. The ASCO guidelines for breast cancer accept that the optimal duration of bisphosphonate therapy is unknown. A recent randomised controlled trial that used bisphosphonates in the adjuvant setting (that is, before bone metastases have developed) found that initial benefits were not maintained once the drug was discontinued.38 Bisphosphonates are ingested by osteoclasts, which subsequently die, removing the drug from the site of bone resorption. This may explain the need to continually reload the bone with bisphosphonate. We recommend that bisphosphonates are continued until no longer clinically relevant. This is supported by ASCO guidelines.30
It was difficult to separate the effect of the drug from the route of administration as the aminobisphosphonates pamidronate and zoledronic acid are given intravenously whereas the principal oral agent was the non-aminobisphosphonate clodronate. We did not find any peer reviewed published studies that provided a direct randomised comparison between oral and intravenous bisphosphonates. One trial directly compared pamidronate with zoledronate and showed no difference in efficacy (study B, R Murphy, Novartis, personal communication).
Intravenous bisphosphonates have better bioavailability than oral bisphosphonates.39,40 The pooled results of trials that used intravenous bisphosphonates were highly significant and mirror the primary analysis. In comparison, oral bisphosphonates showed a significant reduction in only vertebral and non-vertebral fractures, but numbers contributing to the analysis were small. Therefore, at present, most evidence supports the use of intravenous aminobisphosphonates. All bisphosphonates are well tolerated with a low incidence of serious side effects37 and may be administered safely over a period of years.41
Further research is needed to determine the optimum regimen required to treat patients with bone metastases. Clinical trials of bisphosphonates in other disease groups are needed. Results from current studies that are using bisphosphonates in the adjuvant setting to prevent or delay the development of bone metastases in high risk patients are awaited with interest.
Supplementary Material
 Two tables of the included and excluded trials and a full list of references (w1-w98) can be found on bmj.com
Two tables of the included and excluded trials and a full list of references (w1-w98) can be found on bmj.com
We thank members of the project steering group (R A'Hern, R Chinn, D Dearnaley, M Dowsett, S Evans, D Feuer, J Hardy, C Normand, T Powles, D Wonderling) for their guidance. We are grateful to various authors and Novartis Pharmaceuticals who contributed unpublished data.
Contributors: JRR and YS contributed to the design of the study, collected and reviewed the papers, independently extracted the data, analysed the results, and prepared the manuscript for publication. PME supervised JRR and YS, contributed to the design of the study, randomly selected 10% of papers to check, and contributed to the manuscript for publication. SP performed all the statistical analysis and contributed to the design and methodology of the study. KEB developed the original idea, obtained funding, and contributed to the manuscript for publication. SRDJ contributed to the design of the study and interpretation of the results and contributed to the manuscript for publication. JRR is guarantor.
Funding: This review was funded by the NHS Health and Technology Assessment Programme. The conclusions do not necessarily reflect the views of the funding body. The guarantor accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Competing interests: None declared.
References
- 1.Healey JH, Brown HK. Complications of bone metastases. Surgical management. Cancer 2000;88: 2940-51. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Skeletal complications of malignancy. Cancer 1997;80(suppl): 1588-94. [DOI] [PubMed] [Google Scholar]
- 3.Fleisch H. Bisphosphonates: mechanism of action. Endocr Rev 1998;9: 80-100. [DOI] [PubMed] [Google Scholar]
- 4.Francis M, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in-vitro and pathological calcification in-vivo. Science 1969;165: 1264-6. [DOI] [PubMed] [Google Scholar]
- 5.Mundy GR, Yoneda T, Hiraga T. Preclinical studies with zoledronic acid and other bisphosphonates: Impact on the bone microenvironment. Semin Oncol 2001;28(2 suppl 6): 35-44. [DOI] [PubMed] [Google Scholar]
- 6.Russell RG, Rogers M, Frith JC, Luckman SP, Coxon FP, Benford HL, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Min Res 1999;14(suppl 2): 53-65. [DOI] [PubMed] [Google Scholar]
- 7.Luckman SP, Hughes DE, Coxon FP, Russell RG, Rogers MJ. Nitrogen-containing bisphosphonate inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Min Res 1998;13: 581-9. [DOI] [PubMed] [Google Scholar]
- 8.Coxon FP, Helfrich MH, Van't Hof R, Sebti S, Ralston SH, Hamilton A, et al. Protein Geranylgeranylation is required for osteoclast formation, function and survival: inhibition by bisphosphonates and GGTI-298. J Bone Min Res 2000;15: 1467-6. [DOI] [PubMed] [Google Scholar]
- 9.Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, et al. Molecular mechanisms of action of bisphosphonates. Bone 1999;24(5 suppl): 73-9s. [DOI] [PubMed] [Google Scholar]
- 10.Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev 2003;(2): CD002068. [DOI] [PMC free article] [PubMed]
- 11.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309: 1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke M, Oxman AD, eds. Selection bias. Cochrane reviewers' handbook 6.3. Oxford: Cochrane Collaboration, 2001.
- 13.Statacorp. Stata statistical software: release 7.0. College Station, TX: STATA Corporation, 2001.
- 14.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med 1996;334: 488-93. [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. J Clin Oncol 1998;16: 2038-44. [DOI] [PubMed] [Google Scholar]
- 16.Hultborn R, Gundersen S, Ryden S, Holmberg E, Carstensen J, Wallgren UB, et al. Efficacy of pamidronate in breast cancer with bone metastases: a randomized, double-blind placebo-controlled multicenter study. Anticancer Res 1999;19: 3383-92. [PubMed] [Google Scholar]
- 17.Kristensen B, Ejlertsen B, Groenvold M, Hein S, Loft H, Mouridsen HT. Oral clodronate in breast cancer patients with bone metastases: a randomized study. J Intern Med 1999;246: 67-74. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC working party on leukaemia in adults. Br J Haematol 1998;100: 317-25. [DOI] [PubMed] [Google Scholar]
- 19.Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol 1993;11: 59-65. [DOI] [PubMed] [Google Scholar]
- 20.Theriault RL, Lipton A, Hortobagyi GN, Leff R, Gluck S, Stewart JF, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. J Clin Oncol 1999;17: 846-54. [DOI] [PubMed] [Google Scholar]
- 21.Tubiana-Hulin M, Beuzeboc P, Mauriac L, Barbet N, Frenay M, Monnier A, et al. Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases. Bull Cancer 2001;88: 701-7. [PubMed] [Google Scholar]
- 22.Daragon A, Humez C, Michot C, Le Loet X, Grosbois B, Pouyol F, et al. Treatment of mutiple myeloma with etidronate: results of a multicentre double-blind study. Eur J Med 1993;2: 449-52. [PubMed] [Google Scholar]
- 23.Glover D, Lipton A, Keller A, Miller AA, Browning S, Fram RJ, et al. Intravenous pamidronate disodium treatment of bone metastases in patients with breast cancer. Cancer 1994;74: 2949-55. [DOI] [PubMed] [Google Scholar]
- 24.Harris AL, Millward M, Tomkin K, Cantwell BM, Carmichael J, Wilson R, et al. Randomised trial of aminoglutethamide and hydrocortisone with and without disodium pamidronate (APD) in patients with advanced postmenopausal breast cancer and bone metastases. In: Bijvoet OL, Lipton A, eds. Osteoclast inhibition in the management of malignancy-related bone disorders. Seattle, WA: Hogrefe and Huber, 1993: A73.
- 25.Martoni A, Guaraldi M, Camera P, Biagi R, Marri S, Beghe F, et al. Controlled clinical study on the use of dichloromethylene diphosphonate in patients with breast carcinoma metastasizing to the skeleton. Oncology 1991;48: 97-101. [DOI] [PubMed] [Google Scholar]
- 26.Robertson AG, Reed NS, Ralston SH. Effect of oral clodronate on metastatic bone pain: a double-blind, placebo-controlled study. J Clin Oncol 1995;13: 2427-30. [DOI] [PubMed] [Google Scholar]
- 27.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. J Clin Oncol 1998;16: 593-602. [DOI] [PubMed] [Google Scholar]
- 28.Elomaa I, Blomqvist C, Grohn P, Porkka L, Kairento AL, Selander K, et al. Long-term controlled trial with diphosphonate in patients with osteolytic bone metastases. Lancet 1983;1: 146-9. [DOI] [PubMed] [Google Scholar]
- 29.Belch AR, Bergsagel DE, Wilson K, O'Reilly S, Wilson J, Sutton D, et al. Effect of daily etidronate on the osteolysis of multiple myeloma. J Clin Oncol 1991;9: 1397-402. [DOI] [PubMed] [Google Scholar]
- 30.Hillner BE, Ingle JN, Berenson JR, Janjan NA, Albain KS, Lipton A, et al. American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. American Society of Clinical Oncology bisphosphonates expert panel. J Clin Oncol 2000;18: 1378-91. [DOI] [PubMed] [Google Scholar]
- 31.Bishop HM, Cameron DA, Coleman R, Davies AM, Dewar JA, Evans A, et al. British Association of Surgical Oncology Guidelines. Eur J Surg Oncol 1999;25: 3-23.10188849 [Google Scholar]
- 32.Grill V, Martin TJ. Hypercalcaemia of malignancy. Rev Endocr Metab Dis 2001;1: 253-63. [DOI] [PubMed] [Google Scholar]
- 33.Mundy GR. Hypercalcaemia associated with hematologic malignancies. In: Mundy GR, ed. Calcium homeostasis: hypercalcaemia and hypocalcaemia. London: Martin Dunitz, 1990: 100-15.
- 34.Pavoakis N, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2003;(2): CD003474. [DOI] [PubMed]
- 35.Djulbegovic B, Wheatley K, Ross J, Clark O, Bos G, Goldschmidt H, et al. Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev 2003;(2): CD003188. [DOI] [PubMed]
- 36.Dearnaley DP, Sydes MR. Preliminary evidence that oral clodronate delays symptomatic progression of bone metastases from prostate cancer: first results of the MRC Pr05 trial. J Clin Oncol (ASCO) 2001; A693.
- 37.Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al. A systematic review of the role of bisphosphonates in metastatic disease. Health Technol Assess 2003. (in press.) [DOI] [PubMed]
- 38.Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 2002;20: 3219-24. [DOI] [PubMed] [Google Scholar]
- 39.Yakatan GJ, Poyner WJ, Talbert RL, Floyd BF, Slough CL, Ampulski RS, et al. Clodronate kinetics and bioavailability. Clin Pharmacol Ther 1982;31: 402-10. [DOI] [PubMed] [Google Scholar]
- 40.Fitton A, McTavish D. Pamidronate: a review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs 1991;41: 289-318. [DOI] [PubMed] [Google Scholar]
- 41.Ali SM, Hortobagyi G, Harvey H, Seaman J, Knight R, Costa L, et al. Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. J Clin Oncol 2001;19: 3434-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.