Abstract
The envelope glycoprotein (Env) of HIV-1 is displayed on the surface of the virion or infected cell as an oligomer of multiple gp120/gp41 complexes. We sought to unravel the relationships between this oligomeric structure and the requirements for sequential interactions with CD4 and coreceptor (CCR5 or CXCR4). We used a quantitative cell fusion assay to examine the effects of coexpressing pairs of Envs, each nonfunctional because of a specific defect in one of the essential properties. We observed efficient fusion activity upon coexpression of two Env variants, one containing a gp41 subunit with a mutated fusion peptide and the other containing a gp120 subunit with a mutated CD4 binding site or a mismatched coreceptor specificity. We also observed fusion upon coexpression of two Env variants with distinct gp120 defects, i.e., a CD4 binding site mutation and the incorrect coreceptor specificity determinants. Coimmunoprecipitation experiments verified the efficient formation of mixed oligomers, suggesting that the observed fusion reflected subunit complementation within the oligomeric complex. These results support a model in which cooperative subunit interactions within the Env oligomer result in concerted conformational changes upon receptor binding, resulting in activation for fusion. The implications of these findings for Env function and virus neutralization are discussed.
The envelope glycoprotein (Env) of HIV-1 mediates virus entry into target cells by catalyzing a complex series of receptor-binding events and associated conformational changes that result ultimately in fusion between the membranes of the virion and target cell. The biochemical outline of the fusion reaction has been established (reviewed in refs. 1–4). The external gp120 subunit must bind to two distinct receptors on the target cell: CD4 (the “primary receptor”) and a specific chemokine receptor (the coreceptor, usually CCR5 or CXCR4). These receptor interactions trigger the transmembrane gp41 subunit to promote fusion via a process that is presumed to involve insertion of its N-terminal fusion peptide into the membrane of the target cell. A specific sequence of receptor interactions is required to activate the fusion reaction, as revealed by binding analyses with soluble gp120 (5–7), fusion and infectivity experiments with soluble CD4 (8, 9), high-resolution x-ray crystallographic structural determinations (10), and site-directed mutagenesis studies (11). In the favored model, CD4 binding exposes, creates, or stabilizes the coreceptor binding determinants on gp120; interaction with coreceptor triggers additional conformational changes, leading to gp41 activation and consequent fusion.
Env is first synthesized as a high molecular weight precursor designated gp160. The protein forms a homo-oligomer in the endoplasmic reticulum and is transported to the Golgi apparatus where processing by a host cell protease(s) occurs; the functional Env on the surface of both the infected cell and the virion is a homo-oligomer of multiple gp120/gp41 noncovalent complexes. Compelling structural data has defined a gp41 trimeric coiled coil that is believed to represent the receptor-activated postfusion form of the transmembrane subunit; the prefusion form of Env is less clearly defined, with evidence put forth not only for trimers but also for dimers and tetramers (see citations in refs. 1, 3, 4, and 12).
Although the sequential nature of the receptor binding events is well established, little is known about the relationships between the dual receptor requirement and the oligomeric structure of Env. Thus, it is unclear whether every gp120 subunit within an oligomer must interact with both CD4 and coreceptor to activate gp41, or whether a single gp120 bound to CD4 can transmit its activated state in trans to other members of the oligomer; similarly, it is not known whether all gp41 subunits must actively participate for fusion to occur. A related question is whether the individual gp120/gp41 complexes undergo receptor-induced conformational changes independently or cooperatively within the Env oligomer.
We have applied a genetic approach to study these questions. We examined a series of Env variants with defects at specific functional sites in gp120 or gp41 that render the glycoproteins incompetent for fusion with a target cell bearing CD4 and a particular coreceptor. Different combinations of Env variants were coexpressed and tested for complementation in a quantitative gain-of-function cell fusion assay. Our results reveal intriguing higher ordered intricacies of subunit interactions within the HIV-1 Env oligomer that enable functional escape from otherwise debilitating molecular defects.
Materials and Methods
Expression and Function of Envs and Receptors.
HIV-1 Envs and their receptors were produced by using vaccinia virus expression technology, and Env function was quantified with a cell fusion assay based on spectrophotometric quantitation of β-galactosidase production, as described (13). Citations for previously reported plasmid constructs, vaccinia recombinants, and cell lines are given in refs. 8 and 13. Each vaccinia virus was used at a multiplicity of infection of 10.
For the cell fusion assay, target cells were prepared by coinfecting NIH 3T3 cells with vaccinia recombinant vCB21R-LacZ containing the lacZ reporter gene linked to the T7 promotor, plus vaccinia recombinants encoding CD4 (vCB-3) and the designated coreceptor [CCR5, vvCCR5–1107 (14); CXCR4, vCBYF1-fusin] linked to vaccinia early/late promoters. Effector cells were prepared by transfecting HeLa cells with plasmids containing the Env genes linked to a vaccinia early/late promoter and infecting with vaccinia recombinant vP11T7gene1 encoding bacteriophage T7 RNA polymerase. Transfection was performed with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (Boehringer Mannheim); the total amount of DNA was held constant at 5 μg DNA per 25 cm2 flask, in single transfection or cotransfection experiments. Previously described plasmids were used to express wild-type Envs SF162 (plasmid pCB-32) and LAV (plasmid pCB-41). As a negative control, an uncleaveable mutant form of IIIB Env (Unc) was used (plasmid pCB-16). The desired site-directed mutations were introduced into the plasmids encoding wild-type Envs (QuikChange kit, Stratagene): CD4 binding site mutants SF162-BS (plasmid pKS-1) and LAV-BS (plasmid pKS-3); fusion peptide mutants SF162-FP (plasmid pKS-2) and LAV-FP (plasmid pKS-4). The SF162-FP mutant was further modified (ExSite mutagenesis system, Stratagene) to add an 8-aa epitope tag (FLAG, Sigma) to the C terminus of Env, via a 3-aa linker (15); the resulting protein is designated SF162-FP* (plasmid pKS-5). The SF162-BS and SF162-FP mutants were further modified (QuikChange) to introduce a UAG stop codon in place of the Gln at position 701, resulting in deletion of 147 aa from the cytoplasmic tails; the corresponding proteins are designated SF162-BSΔ (plasmid pKS-6) and SF162-FPΔ (plasmid pKS-7). All mutations were verified by sequencing the altered regions, and phenotypes were confirmed for two independently derived plasmid clones.
Coimmunoprecipitation.
Portions of the Env-expressing HeLa effector cells prepared for a parallel cell fusion assay were washed once with PBS and lysed by incubation for 10 min on ice in the same buffer containing 1% Nonidet P-40. The lysates were centrifuged for 10 min in a microcentrifuge to remove insoluble cell debris, and the clarified lysates were evenly divided among separate tubes. Proteins were immunoprecipitated by first incubating with 1 μg of the appropriate mAb for 1 h on ice. Each lysate was immunoprecipitated separately with the broadly cross-reactive T8 anti-gp120 murine mAb (16), and either the anti-FLAG epitope M2 murine mAb (Sigma) or the D47 anti-gp120 V3 loop murine mAb that is specific for T cell line-adapted Env (16). As a control for formation of mixed Env complexes after cell lysis, lysates from cells independently transfected with two different constructs were mixed 1:1 before immunoprecipitation. The resulting immune complexes were precipitated by incubating with 10 μl of a 50% slurry of immobilized protein A/G (Pierce) for 2 h at room temperature followed by centrifugation in a microcentrifuge. The resulting pellets were washed three times with lysis buffer before protein separation by SDS/PAGE (4–20% acrylamide gradient gel) and transfer to nitrocellulose. Protein blots were blocked with 10% milk and probed with 1 μg/ml T8 mAb followed by horseradish peroxidase-conjugated sheep anti-mouse IgG secondary antibody (Boehringer Mannheim) and then detected by chemiluminescence (SuperSignal, Pierce).
Results
Design of Env Variants.
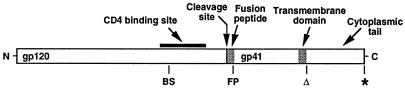
Our experimental approach was to test whether Envs defective in specific functions could complement one another when present within the same oligomer. We used site-directed mutagenesis to produce the desired Env variants in two different strains: SF162, a prototypic CCR5-specific (R5) macrophage-tropic primary strain, and LAV, a prototypic CXCR4-specific (X4) T cell line-adapted strain. The relevant functional regions are shown schematically in Fig. 1. The Env mutants in the CD4 binding site, designated SF162-BS and LAV-BS, each contain a point mutation equivalent to the Asp to Arg substitution originally described at residue 368 of gp120 from the HXBc2 clone (17, 18). This mutation was chosen because the previous studies indicated that it completely abrogates CD4 binding, fusion, and infectivity without affecting the normal transport and processing of the glycoprotein; moreover, x-ray crystallographic analysis has revealed the Asp residue makes a critical contact with CD4 (10). The fusion peptide mutants, designated SF162-FP and LAV-FP, contain a Leu to Arg substitution at residue 26 within the gp41 fusion peptide. This mutation was chosen because it was previously shown to completely abrogate fusion when expressed alone (19), without imparting a dominant negative effect when coexpressed with wild-type glycoprotein (20); the previous studies verified minimal effect of this mutation on glycoprotein maturation and transport. As a third Env variant, we used wild-type Envs that were mismatched for the coreceptor used in a specific experiment. With this set of variants, we could test for complementation of Envs defective in distinct sites required for three critical functions: CD4 binding, coreceptor interaction, and fusion peptide insertion.
Figure 1.
Schematic of HIV-1 Env and variants. The locations of relevant functional domains are shown at the top; genetic modifications are indicated at the bottom, including CD4 binding site mutation (BS), fusion peptide mutation (FP), cytoplasmic tail deletion (Δ), and C-terminal FLAG epitope tag (*).
Complementation Between Env Variants.
To test the ability of fusion-inactive Env variants to functionally complement one another within an oligomeric complex, a gain-of-function cell fusion assay was used (13); this assay closely models the HIV-1 Env-mediated fusion reaction involved in virus entry and infection. Vaccinia virus expression technology was used to express the Env variants on the surface of effector cells (HeLa) and the appropriate receptors on the surface of target cells (NIH 3T3). β-galactosidase produced in response to cell fusion was quantitated spectrophotometrically.
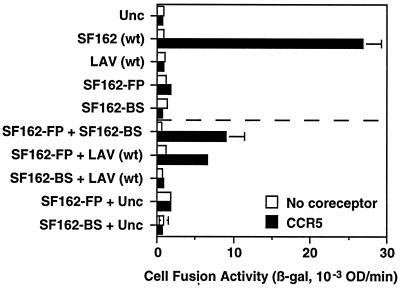
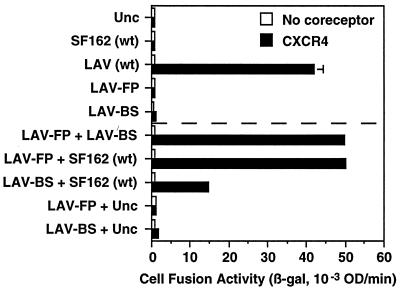
Fig. 2 shows an experiment with target cells expressing CD4 and CCR5; as background controls, parallel assays were performed with target cells lacking coreceptor. Initial experiments assessed the fusion activity for each Env variant expressed alone. As expected, wild-type SF162 Env (R5) yielded robust fusion in this assay. By contrast, no fusion was observed for: the gp41 mutant of SF162 with a defective fusion peptide (SF162-FP), the gp120 mutant of SF162 incapable of binding to CD4 (SF162-BS), or LAV wild type incapable of interacting with CCR5. Also, no fusion activity was observed with the uncleaveable Unc Env control. Interestingly, fusion activity was obtained by coexpressing Env containing the defective fusion peptide with either of the Envs containing nonfunctional gp120 subunits. Thus, coexpression of SF162-FP with SF162-BS gave a significant fusion signal, as did coexpression of SF162-FP with LAV wild type; however, neither SF162 mutant gave a fusion signal when coexpressed with Unc. Typically the fusion levels observed with these complementing Env variants ranged from 25% to 35% of the levels obtained with wild-type SF162. The simplest interpretation is that CD4 and coreceptor binding to the fully functional gp120 subunit on SF162-FP in some way activated the functional gp41 subunit(s) on another member of the oligomer that was associated with a nonfunctional gp120. In contrast to these positive complementation results, Fig. 2 also shows that fusion did not occur upon coexpression of the two Env variants defective in different aspects of receptor interaction, i.e., SF162-BS and LAV wild type, despite the fact that all gp41 subunits were functional.
Figure 2.
Complementation between Env variants for CCR5-dependent fusion. Effector cells expressing the indicated Envs (alone or in combination) were mixed with target cells expressing CD4 and either CCR5 (filled bars) or no coreceptor (open bars). Cell fusion was quantitated by measurement of β-galactosidase activity (β-gal) after 2.5 h. Error bars indicate the standard errors of the mean of duplicate samples. wt, Wild type.
Effect of DNA Transfection Ratio on Complementation Efficiency.
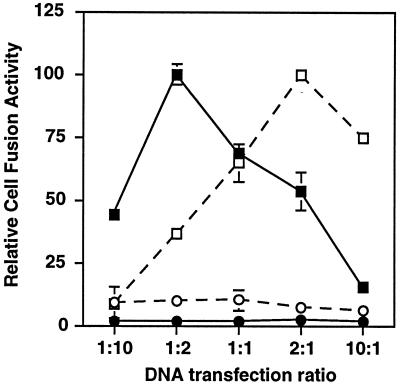
In the experiments presented above, we cotransfected equal amounts of plasmid DNA encoding each Env variant (2.5 μg each, 5 μg total). To determine whether this 1:1 ratio of transfected plasmids was optimal for complementation, we examined the effects of varying the plasmid DNA ratios (keeping the total amount of DNA constant at 5 μg). The results shown in Fig. 3 indicate that fusion was optimal within a DNA transfection ratio range between 1:2 and 2:1. This result supports the suitability of using 1:1 transfection ratios in the complementation studies. At present, we do not know the significance of the minor differences within this range, particularly because the amounts of transfected DNA might not correspond precisely to the amounts of the corresponding expressed proteins.
Figure 3.
Effect of DNA transfection ratio on complementation efficiency. The Env DNA transfection ratios were varied, keeping total amount of DNA at 5 μg. Ratios are shown for SF162-FP:SF162-BS (closed symbols) and SF162-FP:LAV (open symbols). Target cells expressed CD4 and either CCR5 (squares) or no coreceptor (circles). Error bars indicate the standard errors of the mean of duplicate samples.
Verification of Mixed Oligomer Formation.
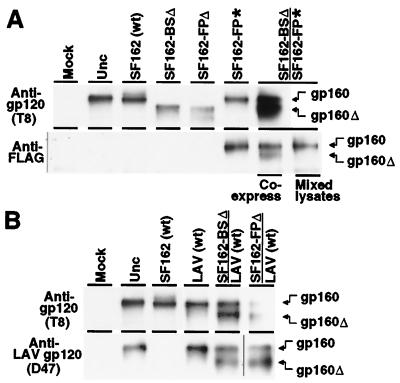
To obtain direct biochemical evidence for mixed oligomer formation in cotransfected cells, we performed coimmunoprecipitation analyses (Fig. 4). Proteins specifically immunoprecipitated from detergent lysates were analyzed by Western blotting, staining with the T8 anti-gp120 mAb (16) which recognizes a conserved epitope present in each Env variant used in this study. For simplicity of detection we analyzed the uncleaved gp160 molecules, which oligomerize in the endoplasmic reticulum during transit to the cell surface (12, 21); a fraction of the molecules escape proteolysis in the Golgi apparatus and thus remain uncleaved. To distinguish the individual Env variants in the immunoprecipitation reactions, we introduced biochemical markers into the cytoplasmic regions: addition of the FLAG epitope tag (*) at the C terminus of one variant to enable specific immunoprecipitation, and deletion of the entire cytoplasmic region (Δ) of the other variant to enable its identification by gel mobility. Each of these modifications has been shown to have negligible effect on the fusogenicity of T cell line-adapted Envs (15, 22). In experiments not shown, we verified that the fusion and complementation activities of the FLAG-tagged and truncated variants closely paralleled the results presented above (Fig. 2) with Envs containing unmodified cytoplasmic regions. As an alternate means of specific immunoprecipitation, an anti-V3 loop mAb (D47) that binds specifically to LAV Env and not to SF162 Env was used to test for coimmunoprecipition of truncated SF162 variants.
Figure 4.
Mixed oligomer formation detected by coimmunoprecipitation of Env variants. Effector cells prepared as in Fig. 2 were lysed in 1% Nonidet P-40, and the lysates were divided into two tubes. HIV-1 Env was immunoprecipitated as designated on the left with either the broadly cross-reactive T8 anti-gp120 mAb, the anti-FLAG epitope tag mAb, or the D47 anti-gp120 mAb that specifically recognizes the V3 loop of LAV Env but not SF162. As a control (mixed lysates), lysates from cells separately transfected with either SF162-BSΔ or SF162-FP* alone were mixed before immunoprecipitation. Immunoprecipitates were analyzed by SDS/PAGE and immunoblotting with the broadly cross-reactive T8 anti-gp120 mAb, as described in Materials and Methods. Another control involved treatment under the identical transfection conditions without DNA (Mock). The symbols Δ and * indicate truncated and FLAG-tagged Envs, respectively. The positions of full-length gp160 (gp160) and truncated gp160 (gp160Δ) are indicated on the right.
Fig. 4A shows the results for coexpression of the truncated SF162-BSΔ and FLAG-tagged SF162-FP*. The upper panel shows the control direct immunoprecipitations with the T8 mAb, to verify the presence of the Env molecules in the lysates from each transfection. The uncleaved Unc mutant was used as a standard to define the position of gp160. Bands at this position were observed for the wild-type SF162 and FLAG-tagged SF162-FP* transfections; the truncated variants SF162-BSΔ and SF162-FPΔ gave faster migrating bands, consistent with the absence of the cytoplasmic regions. In cells transfected with both SF162-BSΔ and SF162-FP*, both bands were observed. The lower panel in Fig. 4A shows the proteins immunoprecipitated with the anti-FLAG mAb. With the lysates from cells transfected with SF162-FP*, a band at the position of gp160 was observed, consistent with direct immunoprecipitation of this FLAG-tagged protein; specificity of the anti-FLAG mAb was confirmed by the absence of bands with each of the Env variants lacking the epitope tag (Unc, wild-type SF162, SF162-BSΔ, and SF162-FPΔ). By contrast, with cells cotransfected with SF162-FP* and SF162-BSΔ, bands corresponding to both proteins were observed, indicating coimmunoprecipitation. To rule out the possibility that this result was a consequence of mixed oligomer formation after cell lysis, a control immunoprecipitation was performed with a mixture of lysates from cells transfected individually with each variant; only the directly immunoprecipitated SF162-FP* variant was observed.
Fig. 4B shows a similar analysis with other pairs of cotransfected Env variants. In this case, the D47 mAb specific for the V3 loop of LAV was used for specific immunoprecipitation. The lower panel verifies the formation of mixed oligomers upon cotransfection of wild-type LAV with either SF162-BSΔ or SF162-FPΔ. The result with the former Env variant shows that the lack of complementation discussed above with SF162-BS and wild-type LAV (Fig. 2) was not due simply to the failure of these variants to form mixed oligomers.
Extension of Complementation Analyses to Other Combinations of Env Variants and Coreceptors.
We wanted to determine whether the complementation activity observed for mutants of the SF162 isolate (R5) fusing with CCR5-expressing targets could be extended to other HIV-1 strains with different coreceptor usage profiles. Therefore, we performed the converse series of experiments, attempting to complement mutations in the LAV strain (X4), using target cells expressing CD4 and CXCR4 (Fig. 5). The expected results were obtained with individual Env variants expressed separately; thus, wild-type LAV Env gave a robust fusion signal, whereas no fusion was detected with either of the two LAV Env mutants LAV-FP (defective fusion peptide) or LAV-BS (defective CD4 binding site), or with wild-type SF162 (incapable of CXCR4 interaction). However, coexpression of LAV-FP with either LAV-BS or wild-type SF162 resulted in complementation of fusion activity. In multiple experiments, the fusion signals obtained with these complementing pairs of Env variants were consistently similar to those obtained with wild-type LAV alone. In contrast with the results shown in Fig. 2, complementation also occurred between the Env variants defective in CD4 binding and coreceptor interaction; thus, a significant (albeit lower) fusion signal was observed upon coexpression of LAV-BS and wild-type SF162.
Figure 5.
Complementation between Env variants for CXCR4-dependent fusion. Effector cells expressing the indicated Envs (alone or in combination) were mixed with target cells expressing CD4 and either CXCR4 (filled bars) or no coreceptor (open bars). Cell fusion was quantitated by measurement of β-galactosidase activity (β-gal) after 2.5 h. Error bars indicate the standard errors of the mean of duplicate samples. wt, Wild type.
We also observed complementation with Env from the dual-tropic (R5X4) 89.6 strain (data not shown); the 89.6-BS mutant complemented the 89.6-FP mutant, on target cells expressing CD4 plus either CCR5 or CXCR4 (data not shown). Thus, complementation between Envs containing defects in distinct functional regions is a general phenomenon not restricted to Envs of a particular phenotype or target cells expressing a particular coreceptor.
Discussion
The studies described herein demonstrate that fusogenic HIV-1 Env can be generated by coexpression of two distinct inactive Env variants, each of which is nonfusogenic when expressed alone (Figs. 2 and 5). The simplest explanation is that the observed activity reflects the functionality of hetero-oligomers in which complementation occurs in trans between individual gp120/gp41 complexes with different defects. This conclusion is supported by the direct biochemical demonstration of mixed oligomer formation (Fig. 4), a phenomenon previously described between different HIV-1 variants (23, 24) as well as between HIV-1 and either HIV-2 or simian immunodeficiency virus (25, 26). Similar complementation approaches have revealed cooperative interactions within mixed oligomers of influenza virus hemagglutinin (27) and Moloney murine leukemia virus envelope glycoprotein (28–30). As in those studies and in view of the likelihood that fusion involves multiple HIV-1 Env oligomers contributing to the formation of a fusion pore (3, 4), we cannot formally exclude the possibility that complementation reflects interactions between different inactive homo-oligomers; however, this interpretation is more difficult to reconcile with the requirement for sequential receptor interactions and associated conformational changes leading to fusion. Another alternative is that reassortment can occur between different gp120/gp41 complexes, i.e., one containing an inactive gp120 subunit associated with a functional gp41 and another containing an active gp120 linked to a defective gp41; a fully functional gp120/gp41 complex would result. This possibility seems unlikely in view of additional observations. First, in contrast with the ability of wild-type LAV Env to complement SF162-FP for CCR5-dependent fusion (Fig. 2), it did not complement SF162 variants containing certain other gp41 mutations (i.e., a different fusion peptide mutant or an N-terminal heptad repeat mutant; data not shown); both of these variants have wild-type gp120 subunits that would be expected to reconstitute a fully functional Env if reassortment occurred. Second, subunit reassortment cannot explain the observed complementation between pairs of Env variants containing different inactive gp120 subunits (LAV-BS + wild-type SF162 for CXCR4-dependent fusion, Fig. 5).
An important conclusion from our studies is that Env function does not require every subunit within the oligomer to be fully functional. Thus, fusion can occur when one (or more?) gp120 subunit(s) is defective for CD4 binding or for coreceptor binding; similarly, an oligomer can function despite the presence of one (or more?) gp41 subunit(s) containing defective fusion peptides. Also, Envs with different coreceptor specificities can function in concert within a mixed Env oligomer. It is important to note that the ability of an oligomer to tolerate the presence of a nonfunctional subunit depends on the precise nature of the defect; certain gp41 mutations have been shown to have trans-dominant effects that abrogate the function of wild-type subunits in the oligomer (20).
Depending on the particular pairs of inactive Envs examined, the complementation activities ranged from 25% to 35% (Fig. 2) to approximately 100% (Fig. 5) of the activities observed with the corresponding fully functional Envs. Numerous experimental variables may contribute to complementation efficiency, including the surface densities of each of the participating proteins, the binding affinities between gp120 and each receptor, the efficiencies of subunit interactions, etc. The findings of constitutive interactions between CD4 and coreceptors may have significance in this regard (14, 31), as may the recent proposal that cooperative interactions involving multiple CCR5 molecules are required for the HIV-1 infection pathway (32). Also of note is that certain complementation effects were not uniformly noted; for example, LAV-BS complemented SF162 wild type for fusion with CXCR4-expressing targets cells (Fig. 5), but the converse activity was not detected (SF162-BS complementation of LAV wild type for fusion with CCR5-expressing cells, Fig. 2). Additional studies are required to determine the various factors that contribute to the efficiency of the observed complementation effects.
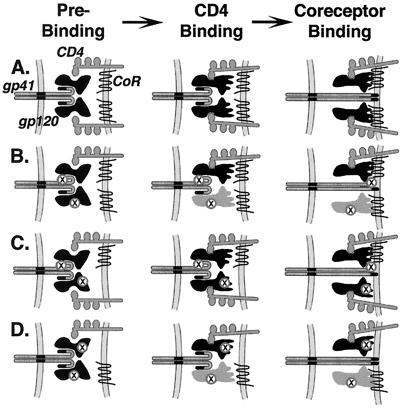
We propose that complementation reflects conformational cross-talk between subunits within the HIV-1 Env oligomer, whereby structural changes induced by CD4/coreceptor binding to one gp120 subunit can be transmitted to other subunits (gp41, and in some cases gp120); moreover, these changes occur in concerted fashion between the multiple gp120/gp41 complexes comprising the oligomer. The schematic models shown in Fig. 6 summarize the observed complementation phenomena and help to conceptualize mechanisms by which cooperative subunit interactions may contribute to coordinated responses upon receptor binding. For example, how might CD4/coreceptor binding to a functional gp120 complexed to a defective gp41 trigger activation of a functional gp41 on another member of the oligomer associated with an inactive gp120 (defective for CD4 binding, Fig. 6B; defective for coreceptor interaction, Fig. 6C)? It is well documented that Env oligomerization is mediated by determinants within the ectodomain of gp41 (1, 3, 4, 12). Thus it can be envisioned not only that binding of an individual gp120 subunit to both CD4 and coreceptor activates the associated gp41 subunit, but also that the activated state can be transmitted by contact to another gp41 subunit(s) within the oligomer. What is the explanation for the observed complementation between Env variants containing gp120 subunits with different functional defects, i.e., one mutated for CD4 binding and the other incapable of coreceptor interaction (Fig. 6D)? Here, too, a plausible model is that the structural signal initiated by CD4 binding to one gp120 subunit is transmitted via gp41–gp41 contacts to another gp120 subunit(s), inducing its association with coreceptor and triggering gp41 activation. Alternatively, recent observations that soluble gp120 can form a stable dimer (33, 34) raises the possibility that the CD4-induced conformational change in one gp120 subunit can be directly transmitted to another contacting gp120 subunit, leading to its induction for coreceptor binding and consequent triggering of the associated gp41 subunits. These latter mechanisms also may contribute to the complementation depicted in Fig. 6B.
Figure 6.
Model for cooperative subunit interactions within the HIV-1 Env oligomer. For simplicity, only two gp120/gp41 complexes are shown. Labeling indicates gp120, gp41, CD4, and coreceptor (CoR); inactive Env regions are designated by X. Different shapes distinguish the preactivated and activated states of gp120; different shading distinguishes the gp120 subunits activated directly by CD4 binding (black) versus indirectly by subunit interactions (gray). (A) Wild-type Env. gp120 subunits on both complexes bind to CD4 and undergo a conformational change to expose determinants critical for coreceptor interaction. Both gp120 subunits bind to coreceptor, leading to activation of both gp41 subunits for fusion peptide insertion. (B) Complementation between one Env variant with a defective fusion peptide and another with a defective CD4 binding site. The gp120 subunit on one complex binds to CD4 and undergoes the conformational change exposing coreceptor interaction determinants. The functional gp41 subunit on the other complex is activated for fusion peptide insertion. We also presume that the coreceptor interaction determinants are indirectly exposed on the other gp120 subunit via concerted subunit interactions and contribute to gp41 activation (as described for D below). (C) Complementation between one Env variant with a defective fusion peptide and another with inactive coreceptor interaction determinants. The gp120 subunits on both complexes bind to CD4 and undergo the associated conformational changes. The coreceptor interaction determinants are functional on only one gp120 subunit; this is sufficient to activate the functional gp41 subunit on the other complex for fusion peptide insertion. (D) Complementation between one Env variant with a defective CD4 binding site and another with inactive coreceptor binding determinants. The gp120 subunit on one complex binds to CD4 and undergoes the associated conformational change. Although the coreceptor binding determinants on this subunit are inactive, cooperative interactions lead to a concerted conformational change in the other gp120 subunit, which then interacts with coreceptor and activates both gp41 subunits for fusion peptide insertion.
The cooperative subunit interactions and concerted conformational changes within the Env oligomer are likely to have important implications for virus neutralization by antibodies. The ability of Env to functionally tolerate the presence of a defective subunit(s) suggests that neutralization may require simultaneous blockade of multiple members of the oligomer; previous studies have indicated exceedingly complex factors influencing the stoichiometric requirements for antibody neutralization (35, 36). Moreover, the concerted nature of the receptor-induced conformational changes may underlie some curious enhancing phenomena sometimes observed with otherwise neutralizing antibodies. An example is the 17b mAb (37), which binds to highly conserved determinants involved in coreceptor binding (6, 10, 11, 38); exposure of the 17b epitope is markedly enhanced upon CD4 binding (37), and the presence of soluble CD4 greatly enhances 17b neutralizing activity (8, 39). Curiously, under certain conditions 17b can significantly enhance infection and fusion (ref. 40 and E. Rosenbaum and E.A.B., unpublished data). Concerted conformational changes within the Env oligomer might underlie these dual effects. At low concentrations, 17b binding to one gp120 subunit might help drive other subunits within the oligomer into an activated conformation with the coreceptor binding determinants exposed, thereby enhancing the fusion process; at high 17b concentration, the coreceptor binding determinant on all subunits within the oligomer would be blocked. These concepts provide a framework for future efforts to delineate the molecular details of the cooperative subunit interactions within the HIV-1 Env oligomer, and to determine their consequences for Env function and its blockade.
Acknowledgments
We thank C. Broder for supplying the vvCCR5–1107 vaccinia virus recombinant, P. Earl for providing the T8 and D47 hybridomas, and R. Center for critical reading of the manuscript. K.S. was supported in part by a National Research Council–National Institutes of Health research associateship. This study was funded in part by the National Institutes of Health Intramural AIDS Targeted Antiviral Program.
Abbreviation
- Env
envelope glycoprotein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230438497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230438497
References
- 1.Wyatt R, Sodroski J. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Chan D C, Kim P S. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 4.Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 5.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. Nature (London) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, et al. Nature (London) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 7.Hill C M, Deng H, Unutmaz D, Kewalramani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzwedel K, Smith E D, Dey B, Berger E A. J Virol. 2000;74:326–333. doi: 10.1128/jvi.74.1.326-333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speck R F, Esser U, Penn M L, Eckstein D A, Pulliam L, Chan S Y, Goldsmith M A. Curr Biol. 1999;9:547–550. doi: 10.1016/s0960-9822(99)80241-3. [DOI] [PubMed] [Google Scholar]
- 10.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Nature (London) 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 12.Doms R W, Lamb R A, Rose J K, Helenius A. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum O, Broder C C, Berger E A. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X D, Wu L, Stantchev T S, Feng Y-R, Ugolini S, Chen H, Shen Z, Riley J L, Broder C C, Sattentau Q J, Dimitrov D S. Proc Natl Acad Sci USA. 1999;96:7496–7501. doi: 10.1073/pnas.96.13.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salzwedel K, West J T, Mulligan M J, Hunter E. J Virol. 1998;72:7523–7531. doi: 10.1128/jvi.72.9.7523-7531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olshevsky U, Helseth E, Furman C, Li J, Haseltine W, Sodroski J. J Virol. 1990;64:5701–5577. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thali M, Olshevsky U, Furman C, Gabuzda D, Li J, Sodroski J. J Virol. 1991;65:5007–5012. doi: 10.1128/jvi.65.9.5007-5012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freed E O, Myers D J, Risser R. Proc Natl Acad Sci USA. 1990;87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed E O, Delwart E L, Buchschacher G L, Jr, Panganiban A T. Proc Natl Acad Sci USA. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earl P L, Moss B, Doms R W. J Virol. 1991;65:2047–2255. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubay J W, Roberts S J, Hahn B H, Hunter E. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poumbourios P, Wilson K A, Center R J, Elahmar W, Kemp B E. J Virol. 1997;71:2041–2049. doi: 10.1128/jvi.71.3.2041-2049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farzan M, Choe H, Desjardins E, Sun Y, Kuhn J, Cao J, Archambault D, Kolchinsky P, Koch M, Wyatt R, Sodroski J. J Virol. 1998;72:7620–7625. doi: 10.1128/jvi.72.9.7620-7625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doms R W, Earl P L, Chakrabarti S, Moss B. J Virol. 1990;64:3537–3540. doi: 10.1128/jvi.64.7.3537-3540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Center R J, Kemp B E, Poumbourios P. J Virol. 1997;71:5706–5711. doi: 10.1128/jvi.71.7.5706-5711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulay F, Doms R W, Webster R G, Helenius A. J Cell Biol. 1988;106:629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Lee S, Anderson W F. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zhu L J, Benedict C A, Chen D G, Anderson W F, Cannon P M. J Virol. 1998;72:5392–5398. doi: 10.1128/jvi.72.7.5392-5398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rein A, Yang C L, Haynes J A, Mirro J, Compans R W. J Virol. 1998;72:3432–3435. doi: 10.1128/jvi.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Lapham C K, Chen H, King L, Manischewitz J, Romantseva T, Mostowski K, Stantchev T S, Broder C C, Golding H. J Virol. 2000;74:5016–5023. doi: 10.1128/jvi.74.11.5016-5023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhmann S E, Platt E J, Kozak S L, Kabat D. J Virol. 2000;74:7005–7015. doi: 10.1128/jvi.74.15.7005-7015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malvoisin E, Kieny M P, Wild F. Virus Res. 1997;49:163–172. doi: 10.1016/s0168-1702(97)01467-6. [DOI] [PubMed] [Google Scholar]
- 34.Center R J, Earl P L, Lebowitz J, Schuck P, Moss B. J Virol. 2000;74:4448–4455. doi: 10.1128/jvi.74.10.4448-4455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonning K, Lund O, Lund O S, Hansen J E S. J Virol. 1999;73:8364–8370. doi: 10.1128/jvi.73.10.8364-8370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzuto C, Sodroski J. AIDS Res Hum Retroviruses. 2000;16:741–749. doi: 10.1089/088922200308747. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan N, Sun Y, Binley J, Lee J, Barbas C F, Parren P W H I, Burton D R, Sodroski J. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]