Abstract
Aims
To investigate the concentration-effect relationship and pharmacokinetics of leflunomide in patients with rheumatoid arthritis (RA).
Methods
Data were collected from 23 RA patients on leflunomide therapy (as sole disease modifying antirheumatic drug (DMARD)) for at least 3 months. Main measures were A77 1726 (active metabolite of leflunomide) plasma concentrations and disease activity measures including pain, duration/intensity of morning stiffness, and SF-36 survey. A population estimate was sought for apparent clearance (CL/F ) and volume of distribution was fixed (0.155 l kg−1). Factors screened for influence on CL/F were weight, age, gender and estimated creatinine clearance.
Results
Significantly higher A77 1726 concentrations were seen in patients with less swollen joints and with higher SF-36 mental summary scores than in those with measures indicating more active disease (P < 0.05); concentration-effect trends were seen with five other disease activity measures. Statistical analysis of all disease activity measures showed that mean A77 1726 concentrations in groups with greater control of disease activity were significantly higher than those in whom the measures indicated less desirable control (P < 0.05). There was large between subject variability in the dose-concentration relationship. A steady-state infusion model best described the pharmacokinetic data. Inclusion of age as a covariate decreased interindividual variability (P < 0.01), but this would not be clinically important in terms of dosage changes. Final parameter estimate (% CV interindividual variability) for CL/F was 0.0184 l h−1 (50%) (95% CI 0.0146, 0.0222). Residual (unexplained) variability (% CV) was 8.5%.
Conclusions
This study of leflunomide in patients using the drug clinically indicated a concentration-effect relationship. From our data, a plasma A77 1726 concentration of 50 mg l−1 is more likely to indicate someone with less active disease than is a concentration around 30 mg l−1. The marked variability in pharmacokinetics suggests a place for individualized dosing of leflunomide in RA therapy.
Keywords: A77 1726, concentration-response, leflunomide, NONMEM, population pharmacokinetics, rheumatoid arthritis
Introduction
Leflunomide is a novel immunomodulatory agent indicated as a disease modifying antirheumatic drug (DMARD) for the treatment of rheumatoid arthritis (RA). Upon oral absorption, it is rapidly converted to the active metabolite, A77 1726 [1–3]. Double-blind, placebo-controlled clinical trials have demonstrated the effectiveness of leflunomide [4–9]. It also has been shown to have comparable efficacy to other established DMARDs such as methotrexate and sulfasalazine [5–9], but except for one study [4], plasma concentrations of A77 1726 were not reported. Some of the withdrawals from these studies could have been due to subtherapeutic or toxic concentrations, as lack of efficacy and adverse events were cited as reasons for withdrawal [4–9].
The mechanism of immunosuppression by leflunomide is affected by several factors, one being the serum concentration of A77 1726 [10]. In a study including 398 RA patients, steady-state plasma A77 1726 concentrations were slightly better predictors of treatment success than the leflunomide dose; the probability of clinical success increased from 25% for placebo to 60% when steady-state A77 1726 concentrations greater than 13 mg l−1 were observed [11].
Although a relationship between plasma A77 1726 concentrations and efficacy in adults with RA appears to exist, current practice does not require therapeutic monitoring and an optimal concentration range for this drug has not been established. A regimen comprising a loading dose of 100 mg once daily for 3 days, followed by a maintenance dose of 20 mg daily is given to patients [12], and long-term maintenance doses greater than 20 mg are not recommended [1, 11]. However, the inflexible, prescriptive nature of this regimen does not take into account interindividual pharmacokinetic variability.
There have been few human pharmacokinetic data on leflunomide reported [1, 13]. The metabolite A77 1726 is extensively protein-bound (99%) and has an apparent volume of distribution (V/F) reported to range from 6 l to 30.8 l, average 12 l [1]. Greater than 90% of a single dose is eliminated, equally via the kidneys (as leflunomide glucuronides and an oxalinic acid derivative of A77 1726), and in the faeces (as A77 1726) [1]. The clearance (CL/F) of A77 1726 following 5–25 mg oral doses was reported to be about 0.020 l h−1, which contributes to a long elimination half-life (2 weeks) in RA patients [1].
The pharmacokinetic data have mostly come from intensive monitoring of drug concentrations over a single dosing interval, performed in both healthy volunteers and patients with RA [1, 13]. These studies provide little information on inter- and intraindividual variability [1, 4]. This can be redressed using a population modelling approach, but until now information on the population pharmacokinetics of leflunomide has only been incompletely published (for example, without values for clearance reported), in abstract form [11, 14, 15].
In view of the variable pharmacokinetics of leflunomide, further studies of the concentration-effect relationship together with more comprehensive population pharmacokinetic analysis may provide a basis for improving the clinical use of this drug. Therefore, the objectives of this study were to investigate the steady-state A77 1726 plasma concentration-response relationship, and to estimate the population pharmacokinetic parameters in RA patients receiving leflunomide as the sole DMARD. We also aimed to identify factors that explain pharmacokinetic variability.
Methods
This study was cross sectional, with A77 1726 concentration and disease activity variables measured at one time in each patient. Patients were also invited to provide two extra plasma samples (taken on separate occasions within the one dosing interval) to enable the population pharmacokinetics of leflunomide to be better characterized. Plasma sampling was predose, 2–3 h postdose, and 6–12 h postdose.
The study recruited subjects attending the outpatient rheumatology clinic at the Princess Alexandra Hospital, Brisbane, Australia, a 600-bed teaching and tertiary referral hospital. Patients from two private rheumatology practices within 100 km of Brisbane also were recruited. All patients attended their clinic for collection of blood for A77 1726 concentration measurement, and for disease assessment. A trained metrologist (VC) who was unaware of the concentration at the time of clinical evaluation performed all measurements of RA-related clinical parameters. The study protocol received prior written approval from the Princess Alexandra Hospital Research Ethics Committee, and the Medical Research Ethics Committee at The University of Queensland (Clearance no. H/261/PHARM/00/PHD). All patients gave written informed consent to the plasma sampling and data collection.
The inclusion criteria for the study were (a) 18 years or older, (b) taking leflunomide for at least 3 months (to ensure steady-state A77 1726 concentrations and sufficient time for efficacy, reported to be 2–3 months [5–7]), (c) RA as diagnosed by the American College of Rheumatology 1987 revised criteria [16] and (d) not receiving other concurrent DMARD therapies within the previous 3 months. Corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) were permitted.
The rheumatological assessments involved recording the number of tender and swollen joints present based on a 28-joint pictorial diagram, patient assessment of duration of morning stiffness (none, <0.5 h, 0.5 to 2 h, >2 h), patient estimate of intensity of morning stiffness (none, mild, moderate, severe), patient assessment of current level of pain (100 mm visual analogue scale) and a measurement of physical and mental health status of the patient using the Medical Outcomes Short Form-36 (SF-36). Patients and their rheumatologists also were asked separately for a global assessment of disease activity (100 mm visual analogue scale).
For the statistical analysis, concentrations of A77 1726 were dichotomized around the median values of the assessments, except for morning stiffness, which was a categorical measure (dichotomized as duration (none/ <0.5 h) and (0.5 to 2 h/ >2 h); intensity (none/mild) and (moderate/severe)). The SF-36 results were summed and transformed into a scoring algorithm using the Windows™ compatible software COES™ (version 2.50; Daw Park, South Australia), with higher scores reflecting better quality of life. The tender and swollen joint counts, pain scores, global assessment of disease activity, and SF-36 summary scores were dichotomized around the median of the results obtained and concentrations of A77 1726 in each of the dichotomized groups were compared. Differences in A77 1726 concentrations between the two groups were evaluated for each measure using the Mann–Whitney U-test and overall using the Wilcoxon signed-rank test. Probability values of less than 0.05 were considered to be statistically significant. The Windows™ compatible analysis software NCSS™ 2004 (Kaysville, Utah) was used for all statistical calculations.
The dose of leflunomide, duration of therapy and time of last dose, together with a list of concurrent therapies, were recorded. Any adverse reactions reported by the patients that might have been related to leflunomide therapy were noted and these reports compared with any notes in their respective medical records for confirmation.
Venous blood samples were collected into 10 ml EDTA tubes for determination of the concentrations of A77 1726. Plasma samples were separated by centrifugation within 1 h of sample collection (500 g, 15 min), and subsequently stored at −20°C until assayed. A77 1726 plasma concentrations were measured using a validated high-performance liquid chromatographic method [17]. Briefly, the assay was accurate, with intra- and interday precision (%CV) <5%. The limit of quantification was 0.8 mg l−1. The absolute recovery of A77 1726 was approximately 100%. Blood samples collected as part of routine clinical care were used in the determination of erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), and C-reactive protein (CRP) concentrations.
Non-linear mixed effects modelling was performed using NONMEM (version V, level 1.1; GloboMax® LLC, Hanover, MD) [18], in conjunction with a Microsoft® FORTRAN 77 compiler (version 1.00). A typical population estimate was sought for CL/F; V/F was fixed to 0.155 l kg−1[15]. A baseline model centred on mean weight (CL/F = θ1, V/F = θ2 × Weight) was developed initially incorporating all patients. Deviations of CL/Fj of the jth individual from the typical (population) value CL/FTV were modelled using an exponential error model, CL/Fj = CL/FTV × eηCL/Fj. Both additive and exponential residual random error models were screened to determine which best described the deviations between model-predicted and observed A77 1726 concentrations.
The following covariates were screened: weight, age, gender, and estimated creatinine clearance (Cockcroft-Gault). Inclusion of the covariates in the population model improved the fit if a decrease in the objective function value (OFV) was observed. The OFV is a NONMEM-calculated goodness-of-fit parameter, and corresponds to minus twice the log likelihood value; a change in the OFV of more than 6.6 for nested models approximates the chi-square distribution at α = 0.01 [18].
The imprecision (expressed as coefficient of variation) of estimation for fixed and random effects parameters was calculated by expressing the asymptotic standard error of estimation as a percentage of the estimated parameter value [18].
Results
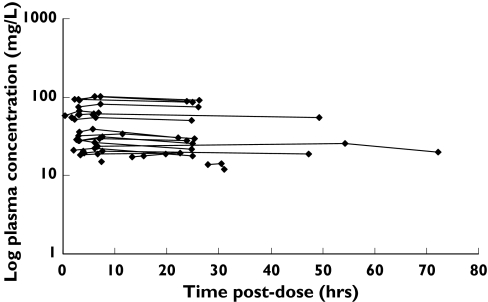
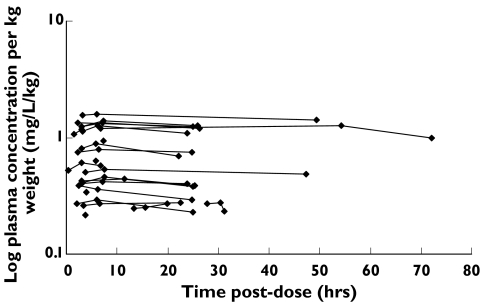
Data were collected from 23 RA patients, and the characteristics of these patients are presented in Table 1. Doses of leflunomide ranged from 5 mg to 20 mg daily, with most receiving 20 mg daily (83%). A total of 59 plasma concentration-time measurements were collected, and there were between one and three plasma concentrations per patient, the majority (74%) providing three samples each. With the exception of eight subjects, all patients provided a plasma sample for each of the requested time periods: predose, 2–3 h postdose, and 6–12 h postdose. The concentration-time profiles were essentially flat with percentage differences (between the highest and the lowest concentrations) for each of the 23 subjects ranging from 2.6% to 13.9%, with a mean ± SD of 7.1 ± 3.5%, and a median of 6.2%. Figure 1 (upper panel) shows the plasma concentration-time profile of A77 1726 for each patient, while the lower panel shows the same data normalized to a 20 mg leflunomide daily dose and normalized per kg body weight. There was negligible linear correlation between leflunomide daily dose and mean steady-state plasma concentration (r2 = 0.08).
Table 1.
Characteristics of study patients (n = 23)
| Mean ± SD | Median | Range | |
|---|---|---|---|
| Demographic data | |||
| Gender (male : female) | 3 : 20 | ||
| Age (years) | 60.7 ± 11.0 | 64 | 33–73 |
| Weight (kg) | 70.2 ± 15.3 | 70 | 44–110 |
| Creatinine clearance (ml min−1) | 81.9 ± 25.8 | 77.5 | 42.7–131.4 |
| Duration of RA (years) | 17.2 ± 11.2 | 14 | 1–42 |
| Duration of LEF therapy (months) | 20.4 ± 15.6 | 17 | 3–60 |
| Concomitant medications | |||
| Corticosteroids | 11 | ||
| NSAIDs | 12 | ||
| Paracetamol | 12 | ||
| Pharmacokinetic data | |||
| Samples | 59 | ||
| Plasma concentrations (mg l−1) | 42.6 ± 27.1 | 29.4 | 11.8–101.5 |
| Postdose sampling time (h) | 14.1 ± 14.9 | 6.75 | 0.3–72.2 |
| Samples per patient | 2.6 ± 0.8 | 3 | 1–3 |
| Dose of leflunomide daily (mg) | 18.1 ± 4.5 | 20 | 5–20 |
Figure 1.
Concentration-time profile of the study population. Upper graph: Observed plasma A77 1726 concentration vs time postdose. Lower graph: Observed plasma A77 1726 concentration (normalized to a 20 mg dose) per kg weight vs time postdose
Results from the nonparametric statistical analyses of each individual disease activity assessment, grouped as mean (±SEM) plasma concentrations of A77 1726 measured in patients, are presented in Table 2. Statistically significantly (P < 0.05) higher plasma A77 1726 concentrations were seen in patients with less swollen joints and with higher SF-36 mental summary scores, than those in whom the measure indicated more active disease. Non-significant trends were also observed in patients with mild or negligible intensity of morning stiffness; with < 0.5 h duration of morning stiffness; with lower global assessment of disease activity score as reported by physician; with lower counts of tender joints; or with < 20 mm h−1 for ESR having higher drug concentrations. Wilcoxon signed-rank analysis also showed that the mean plasma A77 1726 concentrations in groups indicating overall greater control of disease activity were significantly higher than those in whom the disease activity measures indicated less desirable control (P < 0.05).
Table 2.
A77 1726 plasma concentrations (mean ± SEM) and disease activity assessments in RA patients with less (Group 1) or more (Group 2) disease activity. Data were dichotomized around the median values observed for each disease activity measure (except for morning stiffness, which was a categorical measure)
| Mean (±SEM) plasma concentrations (mg l−1) | |||
|---|---|---|---|
| Disease activity measure | Group 1 | Group 2 | P value (Mann–Whitney U-test) |
| Swollen joints* | 52 (± 10) | 30 (± 5) | 0.04 |
| n = 10 | n = 13 | ||
| Tender joints** | 45 (± 8) | 35 (± 7) | 0.21 |
| n = 11 | n = 12 | ||
| Pain*** | 41 (± 10) | 39 (± 6) | 0.78 |
| n = 10 | n = 13 | ||
| Morning stiffness (duration)**** | 44 (± 7) | 29 (± 6) | 0.20 |
| n = 16 | n = 7 | ||
| Morning stiffness (intensity)† | 44 (± 7) | 29 (± 6) | 0.20 |
| n = 16 | n = 7 | ||
| SF-36 Physical summary scores†† | 41 (± 8) | 39 (± 7) | 0.88 |
| n = 12 | n = 11 | ||
| SF-36 Mental summary scores††† | 50 (± 8) | 30 (± 6) | 0.02 |
| n = 11 | n = 12 | ||
| Patients global assessment†††† | 48 (± 9) | 32 (± 5) | 0.35 |
| n = 11 | n = 12 | ||
| Physicians global assessment§ | 49 (± 9) | 31 (± 5) | 0.26 |
| n = 11 | n = 12 | ||
| ESR§§ | 44 (± 9) | 35 (± 8) | 0.20 |
| n = 11 | n = 10 | ||
| RF§§§ | 37 (± 9) | 37 (± 6) | 0.92 |
| n = 8 | n = 14 | ||
Wilcoxon signed-rank test indicates Group 1 and 2 are significantly different (P = 0.003).
Group 1: * < 5 joints; ** < 8 joints; *** < 20 mm; **** < 0.5 h; † none/mild; †† > 36; ††† > 45; †††† < 24.5 mm; § < 38.5 mm; §§ < 20 mm h−1; §§§ negative
Group 2: * > 5 joints; ** > 8 joints; *** > 20 mm; **** > 0.5 h; † moderate/severe; †† < 36; ††† < 45; †††† > 24.5 mm; § > 38.5 mm; §§ > 20 mm h−1; §§§ positive
A statistically significant difference (P < 0.05) was observed only in patients with better SF-36 mental summary score when the data were dichotomized an alternative way, around the midpoint of the measures (i.e. 50 mm for the pain scale), rather than around the median measured values. However, the mean plasma A77 1726 concentration in those groups with better control of disease activity was significantly greater than in those with more active disease even when endpoints were dichotomized around their midpoints (P < 0.05).
There were 14 patients who had adverse effects related to leflunomide use. The most frequent were gastrointestinal disturbances and diarrhoea, alopecia, and rash. Previous abnormalities in liver function tests (LFT) were in medical records of some patients, but all LFT were normal at the time of study. Mean A77 1726 plasma concentration in patients experiencing adverse reactions (37 ± 26 mg l−1) was not significantly different from those of patients reporting no adverse reactions (44 ± 28 mg l−1; P > 0.05, Mann–Whitney U-test).
A ‘steady-state infusion’ model with exponential residual random error best described the pharmacokinetic data. In model building, age significantly reduced the OFV by 8 units when compared against the base model, posthoc empirical Bayesian estimates of CL/F decreased linearly with increasing age, while the residual variability remain unchanged at 8.5%.
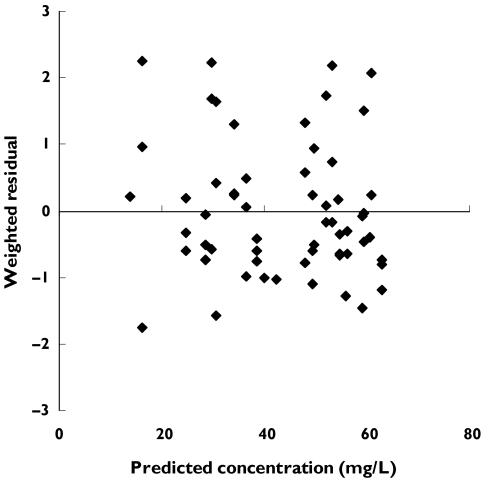
Figure 2 shows a plot of measured A77 1726 vs population model-predicted concentrations for the final model. In the plot of weighted residual vs population model-predicted concentrations (Figure 3), the majority of deviations of the population model from the data were within 2 units of the zero ordinate. The typical parameter estimate for CL/F was 0.0184 l h−1 with considerable interindividual variability (CV = 50%). The 95% confidence interval (CI) was 0.0146–0.0222 l h−1. The residual variability (% CV) was 8.5%. A summary of the final population pharmacokinetic model parameter values is provided in Table 3.
Figure 2.
Observed vs model-predicted A77 1726 plasma concentrations obtained from final model. The solid line represents the line of identity
Figure 3.
Weighted residuals vs model-predicted A77 1726 plasma concentrations obtained from final model
Table 3.
Final population pharmacokinetic model of A77 1726 in RA patients
| Parameter estimation | Value | Precision of estimate (% CV) |
|---|---|---|
| Structural model | ||
| CL/F (l h−1) = θ1 × (1 + θ3 × (AGE−60)) | ||
| V/F (l) = θ2 × WT | ||
| θ1 | 0.0184(0.0146, 0.0222)† | 10.7 |
| θ2 | 0.155* | |
| θ3 | −0.0216(−0.0383, −0.0049)† | 39.5 |
| Variance model | ||
| Inter-individual variability in CL/F (%) | 50.2 | 25.5 |
| Residual random error (%) | 8.5 | 21.3 |
WT: Weight.
Fixed in all modelling.
95% confidence interval.
Discussion
This study supported the existence of a concentration-effect relationship for leflunomide in RA. Plasma concentration of A77 1726 of 50 mg l−1 was more likely to indicate someone with less active disease than a concentration around 30 mg l−1. Improved RA, measured by fewer swollen joints and higher SF-36 mental summary scores, was associated with statistically significant higher plasma concentrations and there were trends observed in other measures. In addition, results from all 11 individual disease activity assessments indicated that overall the groups with greater control of the disease had significantly higher plasma A77 1726 concentrations.
It is likely that because of the small number of patients, only large differences could be observed. However, it is the large differences that are of most interest to clinicians when individualizing doses and looking for true clinical differences related to drug concentration. A statistical correction can be applied to account for the multiple hypothesis testing, but while this is of interest statistically, it is the large clinical differences which are of relevance in these data.
A lack of sufficient sensitivity in some of the measures might blunt some differences. For example, the visual analogue scale (VAS) for pain assessment, while easy to administer, can be hindered by the ability of the patient to assess precisely their level of pain at one point in time on a 100 mm scale [19–21]. Such a scale may be more useful in a time sequence, observing relative changes in pain control.
The generic nature of the SF-36 health survey is not sensitive to small differences [22]. However, it does enable an overall assessment of the disease by the patient [22] and again, it may be more useful in a time sequence, looking at relative changes in quality of life outcomes with therapy (and drug concentration).
Fifty percent of the patients were receiving concomitant corticosteroids, NSAIDs, or paracetamol (22% received the triple combination), which may influence disease activity measures. However, comparison of the disease activity measures between the two groups (receiving/not receiving the concomitant drugs) for each of the three drugs did not yield any significant differences (P > 0.05, Mann–Whitney U-test), indicating a lack of confounding.
Our results provided some indications that improved control of RA could be related to higher plasma concentrations of A77 1726. However, there was no obvious relationship between concentration and toxicity. Due to the low number of such events, the data were analyzed in a gross, nonspecific way using a dichotomous approach of adverse event/no adverse event, and plasma concentrations were not collected at the actual times the patients were experiencing these adverse events. Furthermore, in a recent case report, a patient accidentally ingested 120 mg leflunomide daily for 1 month and presented with plasma concentration of 100 mg l−1, yet experienced no adverse reactions [25]. The absence of a clear limit to which the concentration can be increased is an inherent problem with studies of cross-sectional design, and dosage recommendations are difficult to define from the results. This is a hypothesis-generating study, as opposed to hypothesis testing. As such, a prospective study evaluating whether achievement of higher concentrations actually does lead to better outcomes would be the next step. We hypothesize that achieving plasma concentrations of A77 1726 of 50–60 mg l−1 would lead to lower disease activity than concentrations of 30 mg l−1, or less.
The average daily dose was 18 mg with the majority of patients taking the current recommended guideline of 20 mg, once daily [12]. A recent report suggested that doses up to 40 mg daily increased the effectiveness of leflunomide therapy, and can be considered in patients exhibiting subclinical response to 20 mg daily [24]. However, this is still an empirical result and it is more likely, from our data, that dose individualization targeting specific concentrations would provide more efficacy, rather than an empirical doubling of dose, which could be accompanied by an increased risk of adverse events in a proportion of the population.
The population estimate of CL/F for A77 1726 (0.0184 l h−1), derived from the final pharmacokinetic model with age as a covariate, was similar to the mean value (0.022 l h−1) reported in a brief abstract of a previous population kinetic study [15]. The A77 1726 plasma concentrations were reported to be analyzed using a one-compartment model in two other studies, but details were absent in these abstract reports [14, 15]. However, an apparent ‘steady-state infusion’ model had to be used for describing the disposition of A77 1726 in the present study because of difficulty in estimating the first-order absorption rate constant (Ka) and volume of distribution due to lack of absorption phase and distribution phase data. The combination of short dosing intervals and long half-life contributed to the plateau-effect observed in the concentration-time profiles, and mimicked the profiles typically seen with drugs administered as a constant infusion (Figure 1).
Inclusion of age as a covariate for clearance, although significant in reducing the OFV, did not decrease the variability considerably (56–50%). Other covariates, including weight, failed to reduce the interindividual variability associated with CL/F. The patient weights were quite similar, ranging from 50 to 80 kg. Shi et al.[14], who studied three different patient groups, including children, with wider weight ranges (10–19.9 kg, 20–40 kg, and > 40 kg), concluded that leflunomide dosage should be adjusted according to weight.
For a pharmacokinetic study that utilizes cross-sectional data, limitations are inherent. Factors responsible for the large interindividual variability in CL/F remain unexplained. It is likely that other covariates such as concomitant medication would explain some variability for A77 1726, but only one or a few patients used each concomitant therapy. Evaluation of posthoc clearance against concomitant medications failed to detect the influence of any particular drugs.
It was necessary to fix V/F per kg to an average value reported previously [15], as attempts to estimate volume failed due to the flat concentration-time profiles which gave very limited information about both volume and absorption rate parameters. In terms of long-term dosing and dosage predictions with leflunomide, CL/F and not V/F is the relevant pharmacokinetic parameter.
Including patients on combination DMARD therapies, with leflunomide, might increase the amount of available data to characterize better A77 1726 kinetics in future studies. The value of the specific data reported here is that they were collected without these added confounders as no patient was receiving other concomitant DMARDs. In clinical practice, combination DMARD therapy is now routine and future studies will have to take account of these as covariates (for kinetic studies) or as confounders (for dynamic studies).
The results of this study of leflunomide in RA patients indicated a concentration-effect relationship. Patients with concentrations of A77 1726 of around 50 mg l−1 had less active disease measures than those with concentrations around 30 mg l−1. However, this hypothesis remains to be tested in a prospective study. Furthermore, a population pharmacokinetic profile of A77 1726 was generated. The marked pharmacokinetic variability suggests a place for more flexible dosing of leflunomide, targeting specific plasma concentrations of A77 1726 in RA therapy.
Acknowledgments
This work was financially supported by a grant funded by the National Health and Medical Research Council (#210173). The authors gratefully thank Dr Peter Nash, Dr Julien de Jager, Dr Phillip Vecchio, and their clinics for assistance throughout the study.
References
- 1.Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet. 2002;41:421–30. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Leflunomide Aventis Pharma. Curr Opin Invest Drugs. 2001;2:222–30. [PubMed] [Google Scholar]
- 3.Fox RI. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol Suppl. 1998;25(Suppl 53):20–6. [PubMed] [Google Scholar]
- 4.Mladenovic V, Domljan Z, Rozman B, Jajic I, Mihajlovic D, Dordevic J, Popovic M, Dimitrijevic M, Ziukouic M, Campion G. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995;38:1595–603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- 5.Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, Fox R, Moreland L, Olsen N, Furst D, Caldwell J, Kaine J, Sharp J, Hurley F, Lowe-Friedrich I. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigation Group. Arch Intern Med. 1999;159:2542–50. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Kalden JR, Scott DL, Rozman B, Kvien TK, Larsen A, Loew-Friedrich I, Oed C, Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353:259–66. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- 7.Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gomor B, Van Den Bosch F, Nordstrom D, Bjorneboe O, Dahl R, Horslev-Petersen K, Rodriguez De La Serna A, Molloy M, Tikly M, Oed C, Rosenburg R, Loew-Friedrich I. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000;39:655–65. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, Furst D, Sharp J, Moreland L, Caldwell J, Kaine J, Strand V. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of leflunomide in the treatment of rheumatoid arthritis Trial Investigation Group. Arthritis Rheum. 2001;44:1984–92. doi: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Scott DL, Smolen JS, Kalden JR, van de Putte LB, Larsen A, Kvien TK, Schattenkirchner M, Nash P, Oed C, Loew-Friedrich I. Treatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann Rheum Dis. 2001;60:913–23. doi: 10.1136/ard.60.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong AS, Huang W, Liu W, Luo J, Shen J, Xu W, Ma L, Blinder L, Xiao F, Xu X, Clardy C, Foster P, Williams JA. In vivo activity of leflunomide: pharmacokinetic analyses and mechanism of immunosuppression. Transplantation. 1999;68:100–9. doi: 10.1097/00007890-199907150-00020. [DOI] [PubMed] [Google Scholar]
- 11.Weber W, Harnisch L. Use of a population pharmacokinetic model to predict clinical outcome of leflunomide, a new DMARD in the treatment of rheumatoid arthritis [Abstract] Arthritis Rheum. 1997;40(Suppl 9):s153. [Google Scholar]
- 12.Infante R, Lahita RG. Rheumatoid arthritis. New disease-modifying and anti-inflammatory drugs. Geriatrics. 2000;55:30–2. 35–6,39–40. [PubMed] [Google Scholar]
- 13.Li J, Yao HW, Jin Y, Zhang YF, Li CY, Li YH, Xu SY. Pharmacokinetics of leflunomide in Chinese healthy volunteers. Acta Pharmacol Sin. 2002;23:551–5. [PubMed] [Google Scholar]
- 14.Shi J, Kovacs SJ, Ludden TM, Bhargava V. Population pharmacokinetics (PPK) analysis of A77 1726 (M1) after oral administration of leflunomide (LEF) in pediatric subjects with polyarticular course juvenile rheumatoid arthritis (HRA) [Abstract] Clin Pharmacol Ther. 2004;75:P5. [Google Scholar]
- 15.Weber W, Harnisch L. 11th European League Against Rheumatism (EULAR) Symposium. Geneva, Switzerland: 1998. Leflunomide in rheumatoid arthritis: Population pharmacokinetic analysis of phase III studies [Abstract] p. 158. September 5–8. [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Chan V, Charles BG, Tett SE. Rapid determination of the active leflunomide metabolite A77 1726 in human plasma by high-performance liquid chromatography. J Chromatogr B. 2004;803:331–5. doi: 10.1016/j.jchromb.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 18.NONMEM Project Group. NONMEM Users Guide. San Francisco: University of California; 1992. [Google Scholar]
- 19.Linton SJ, Gotestam KG. A clinical comparison of two pain scales: correlation, remembering chronic pain, and a measure of compliance. Pain. 1983;17:57–65. doi: 10.1016/0304-3959(83)90127-6. [DOI] [PubMed] [Google Scholar]
- 20.Duncan GH, Bushnell MC, Lavigne GJ. Comparison of verbal and visual analogue scales for measuring the intensity and unpleasantness of experimental pain. Pain. 1989;37:295–303. doi: 10.1016/0304-3959(89)90194-2. [DOI] [PubMed] [Google Scholar]
- 21.Clark P, Lavielle P, Martinez H. Learning from pain scales: patient perspective. J Rheumatol. 2003;30:1584–8. [PubMed] [Google Scholar]
- 22.Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. Br Med J. 1996;312:1016–20. doi: 10.1136/bmj.312.7037.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists. Pharmaceutical Society of Australia Royal Australian College of General Practitioners. Australian Medicines Handbook 2004. 5th edn. Adelaide: Australian Medicines Handbook Pty Ltd; 2004. [Google Scholar]
- 24.Fiehn C, Rochel E, Ho AD, Max R. Dose escalation of leflunomide (LEF) to 40 mg once daily in patients with rheumatoid arthritis and insufficient response to standard dose LEF [Letter] Ann Rheum Dis. 2004;63:746–7. doi: 10.1136/ard.2003.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamali S, Kasapoglu E, Uysal M, Inanc M, Gul A. An unusual overdose of leflunomide in a patient with rheumatoid arthritis [Letter] Ann Pharmacother. 2004;38:1320–1. doi: 10.1345/aph.1D606. [DOI] [PubMed] [Google Scholar]