Abstract
An increase in transmitter release accompanying long-term sensitization and facilitation occurs at the glutamatergic sensorimotor synapse of Aplysia. We report that a long-term increase in neuronal Glu uptake also accompanies long-term sensitization. Synaptosomes from pleural-pedal ganglia exhibited sodium-dependent, high-affinity Glu transport. Different treatments that induce long-term enhancement of the siphon-withdrawal reflex, or long-term synaptic facilitation increased Glu uptake. Moreover, 5-hydroxytryptamine, a treatment that induces long-term facilitation, also produced a long-term increase in Glu uptake in cultures of sensory neurons. The mechanism for the increase in uptake is an increase in the Vmax of transport. The long-term increase in Glu uptake appeared to be dependent on mRNA and protein synthesis, and transport through the Golgi, because 5,6-dichlorobenzimidazole riboside, emetine, and brefeldin A inhibited the increase in Glu uptake. Also, injection of emetine and 5,6-dichlorobenzimidazole into Aplysia prevented long-term sensitization. Synthesis of Glu itself may be regulated during long-term sensitization because the same treatments that produced an increase in Glu uptake also produced a parallel increase in Gln uptake. These results suggest that coordinated regulation of a number of different processes may be required to establish or maintain long-term synaptic facilitation.
The sensorimotor synapse of Aplysia has proven particularly useful for the study of the cellular and molecular mechanisms of long-term facilitation as well as several other types of neural plasticity (1–3). Thus far, several neuronal properties have been implicated in long-term memory, including modulation of membrane currents, regulation of transmitter release, and changes in morphology (4–11). All of these long-term changes are dependent on both transcription and translation (5, 12, 13). Given that transmitter release is increased during long-term facilitation, one question that arises is whether other long-term presynaptic changes such as transmitter uptake and synthesis are coordinated with increased transmitter release.
An increasing body of evidence indicates that the excitatory transmitter of the sensorimotor synapse is Glu (14–19). Glu transporters are poised to impact synaptic efficacy significantly because the uptake of transmitter represents a major mechanism whereby neurotransmission is terminated and neurotransmitter is recycled (20–22). Inhibiting basal Glu uptake affects the amplitude and duration of postsynaptic potentials and currents at a number of different synapses including the sensorimotor synapse of Aplysia (19, 23–27). In addition, blockade of Glu uptake immediately after aversive training blocks the expression of long-term memory in the newborn chick (28). These findings demonstrate that Glu uptake is important for normal synaptic function and suggest Glu uptake may be involved in expression of plasticity at glutamatergic synapses. Thus, we hypothesized that the increased release of transmitter at the sensorimotor synapse during facilitation is accompanied by an increase in Glu uptake.
Materials and Methods
Aplysia californica (100–150 g) were obtained from Marinus (Long Beach, CA) and Alacrity Marine Biological (Redondo Beach, CA). They were maintained at 15°C under 12-h light/12-h dark and fed every 2–3 days. Animals were in the lab for 3 days before use. Experiments investigating duration of siphon withdrawal after long-term sensitization training or exposure to 5-hydroxytryptamine (5-HT; serotonin) in vivo were performed as described (6, 11).
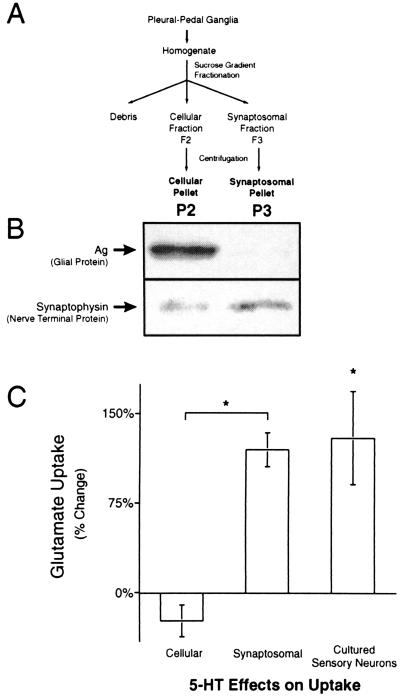
Uptake was measured by using a synaptosomal preparation derived from pleural-pedal ganglia as described (Fig. 3A; ref. 29). Animals were anesthetized with an injection of isotonic MgCl2. Pleural-pedal ganglia were removed and trimmed of excess connective tissue. Synaptosomes were immediately prepared as the uptake activity declined with time of ganglia in culture. Each experiment involved synaptosomes from 8–10 ganglia. In experiments on Na+ dependency of uptake, the Na+ of the Ca2+-free seawater [460 mM NaCl/10 mM KCl/55 mM MgCl2/20 mM Tris⋅HCl (pH 7.4)/0.1% glucose] was completely replaced with N-methyl-d-glucamine (Sigma).
Figure 3.
Increases in Glu uptake were caused by neuronal Glu transporters. Glu uptake in two fractions generated by the synaptosomal isolation technique and in cultured sensory neurons was studied to determine if increases in Glu uptake were neuronal or glial. (A) A flow chart of the isolation technique for the synaptosome (P3) and cellular (P2) preparations. (B) Immunoblots of P2 (Left) and P3 (Right) preparations for Ag, a glial protein, and synaptophysin, a nerve terminal protein. Ag is at least tenfold enriched in the cellular (P2) relative to the synaptosomal (P3) preparation (n = 3). Synaptophysin is enriched at least threefold in the P3 relative to the P2 preparation (n = 4). Only one immunoreactive band was seen for both Ag and synaptophysin. Analysis of the Coomassie-stained transferred gel was used to verify equal protein loading. (C) Glu uptake in the P2 and P3 preparations 24 h after treatment in vivo with 500 μM 5-HT. Glu uptake was increased in the synaptosomal (n = 8), but not the cellular preparation (n = 5). There was a significant increase in Glu uptake by cultured sensory neurons 24 h after treatment with 5-HT (n = 4).
Uptake was determined by incubation of 15–30 μg of synaptosomal protein for 20 min in Glu ([U-14C], ≥250 mCi/mmol, ICN), Gln ([U-14C], ≥200 mCi/mmol, NEN) or Leu ([2,3,4,5-3H], ≥110 Ci/mmol, ICN). Experiments were terminated by dilution of the synaptosomes with 10 volumes of ice-cold Ca2+-free seawater. Synaptosomes were then centrifuged (16,000 × g) for 5 min. The pellet was rinsed three times with 4°C Ca2+-free seawater and dissolved directly in scintillation fluid to determine total Glu uptake. Uptake was normalized to total protein.
The enrichment of synaptosomes was verified via immunoblot of synaptophysin, a nerve terminal protein (30), and Ag, a glial protein (31). Whole fractions and their pellets were solubilized in 1% SDS and diluted in a 3X loading dye (Bio-Rad). Proteins were separated by 12% SDS/PAGE, transferred to a poly(vinylidene difluoride) (Bio-Rad) membrane, blocked with 5% dry milk (Carnation, Glendale, CA), and blotted with either a mAb against synaptophysin (SY38, 0.7 μg/ml, Boehringer Mannheim) or an affinity-purified polyclonal antiserum against Ag (fraction 4, 1:5,000, a gift from I. Levitan, University of Pennsylvania, Philadelphia). Immunoreactivity was visualized with a chemiluminescent detection system (enhanced chemiluminescence, Amersham International). The horseradish peroxidase-conjugated secondary antibodies used (Jackson ImmunoResearch, 1:20,000) were donkey anti-mouse (Synaptophysin) or donkey anti-rabbit (Ag).
Ag is a secreted glial protein in Aplysia (31). To ensure that the immunoblotted Ag was contained within glia or glial fragments, all fractions were exposed to proteinase K [Sigma; 0.125 mg/ml in artificial seawater: 395 mM NaCl/28 mM Na2SO4/10 mM KCl/50 mM MgCl2/10 mM CaCl2/10 mM Tris⋅HCl (pH 8)] to eliminate extracellular protein. The synaptosomal fractions were diluted with an equal volume of proteinase K solution, and incubated at 20°C for 0.5 h. The reaction was then incubated at 4°C for 15 min with PMSF (5 mM, Sigma) to inactivate proteinase K.
Uptake of Glu by synaptosomes was characterized with Glu uptake inhibitors: dl-threo-β-hydroxyaspartate (THA, Sigma), pyrrolidine-dicarboxylic acid (Research Biochemicals, Natick, MA), and aminocyclobutane-dicarboxylic acid (Sigma). All drugs were dissolved in 1 N NaOH to a final concentration of: 134 mM THA/628 mM pyrrolidine-dicarboxylic acid/251 mM aminocyclobutane-dicarboxylic acid. Final solutions were made by adding the drug solution and an equal volume of 1 N HCl to Ca2+-free seawater in which the salt concentrations and pH were modified to compensate for the addition of the volume of drug solution.
Glu uptake was measured in sensory neurons cultured as described (32, 33) at a density of 20–70 neurons per dish. Neurons were allowed to grow for 5 days before use in a medium consisting of equal parts isotonic L-15 and hemolymph. To measure uptake, the medium was replaced with artificial seawater [395 mM NaCl/28 mM Na2SO4/10 mM KCl/50 mM MgCl2/10 mM CaCl2/30 mM Hepes (pH 7.65)]. After rinsing with artificial seawater, cultures were incubated in 2 ml of 10 μM [14C]Glu at 15°C for 30 min. Uptake was terminated by rinsing in 4°C seawater. Excess solution was quickly removed with a KimWipe, and cells and their processes were lifted from the culture plate with scintillation fluid. Glu uptake was normalized to the number of sensory neurons on the culture dish.
Glu uptake was also studied in groups of 10 isolated pleural-pedal ganglia. After trimming, pleural-pedal ganglia were randomized and placed into a solution consisting of two parts isotonic L-15, one part hemolymph, and one part buffered seawater [Instant Ocean seawater with 30 mM Hepes (pH 7.65)] (32) for 2 h before the experimental treatments began.
Data on duration of siphon withdrawal 24 h after treatment with 5-HT, Glu uptake after treatment with 5-HT and electrical stimulation (Fig. 2), and the effects of brefeldin A on Glu uptake (Fig. 6) were analyzed with a one-way ANOVA. Post hoc analysis was performed by using the T-Method of unplanned comparisons (Fig. 2), and the Tukey-Kramer procedure (Figs. 4A, 5, and 6). Fractional differences in the increase in uptake caused by 5-HT treatment were analyzed by using Student's t test (Fig. 3C). Comparison of Lineweaver–Burk plots was done with an analysis of covariance (Fig. 1). In all tests, significance was set at P ≤ 0.05.
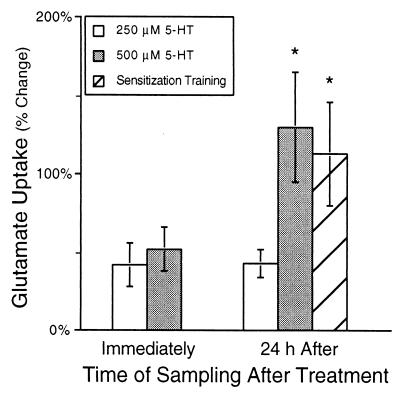
Figure 2.
Glu uptake is regulated by 5-HT and sensitization training. Glu uptake was measured from synaptosomes immediately and 24 h after a 1.5-h treatment in vivo with either 250 or 500 μM 5-HT, or long-term sensitization training (electrical stimulation). Immediately after treatment with either 250 (n = 7) or 500 μM 5-HT (n = 4), Glu uptake was modestly increased. However, 500 μM 5-HT (n = 5) and sensitization training (n = 5) had a large, significant effect on uptake 24 h after treatment. The effects of 500 μM 5-HT and sensitization training were significantly larger than the effect of 250 μM 5-HT 24 h after treatment (n = 4). All increases in Glu uptake were significantly different from no change. An asterisk (*) indicates the change in uptake was significantly (P < 0.05) different from the effect of 250 μM 5-HT.
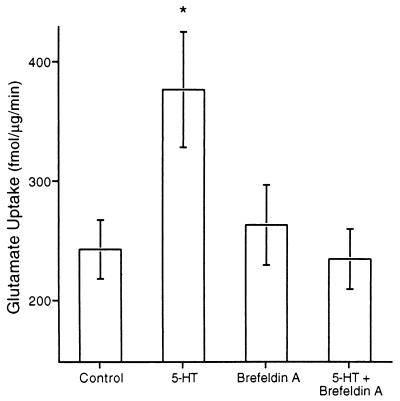
Figure 6.
Brefeldin A blocks the long-term increase in Glu uptake. Synaptosomal Glu uptake was significantly increased 24 h after treatment of pleural-pedal ganglia with 5-HT in vitro (n = 7). Exposure to brefeldin A (18 μM) for 24 h, beginning immediately after the treatment blocked the increase in Glu uptake (n = 5). Brefeldin A treatment alone had no effect on Glu uptake (n = 5).
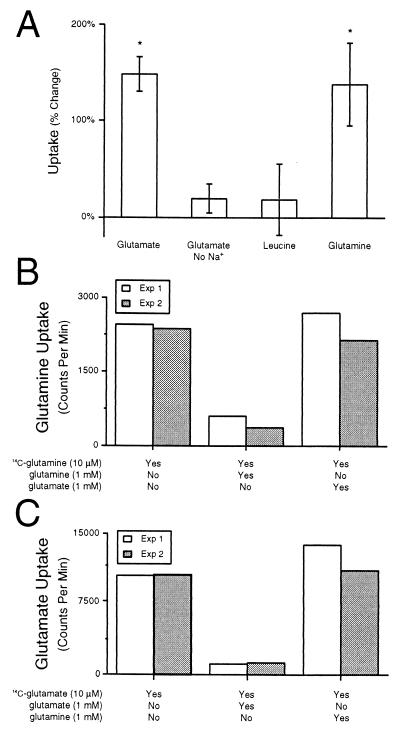
Figure 4.
Specificity of increases in Glu uptake. (A) The increase in synaptosomal Glu uptake produced by 500 μM 5-HT (n = 12) was not observed when sodium was removed from the extracellular media (n = 6). Synaptosomal uptake of Leu was not affected by 5-HT in vivo (n = 4). Synaptosomal Gln uptake was significantly increased by 5-HT in vivo (n = 6). (B) Gln uptake was inhibited by excess Gln, but not by excess Glu, indicating that Gln uptake was through a Gln transporter. (C) Glu uptake was inhibited by excess Glu, but not by excess Gln, indicating that Glu uptake was through a Glu transporter.
Figure 5.
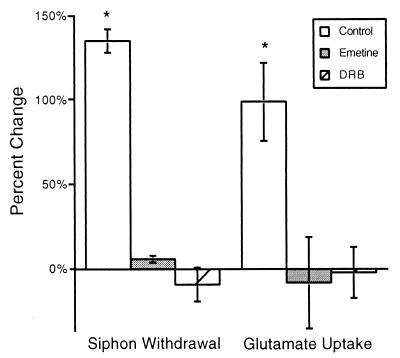
Emetine and DRB block long-term sensitization and the long-term increase in Glu uptake. Electrical stimulation applied to one side of an animal induced long-term sensitization of the siphon withdrawal reflex 24 h after stimulation (F = 12.98, df = 13, P < 0.0005). Injection of emetine (n = 3) or DRB (n = 3) into Aplysia 30 min before electrical stimulation significantly inhibited the appearance of long-term sensitization (P < 0.05). The long-term increase in Glu uptake produced by electrical stimulation (n = 5) was significantly inhibited by injection of emetine (n = 4, P < 0.05) or DRB (n = 3, P < 0.05).
Figure 1.
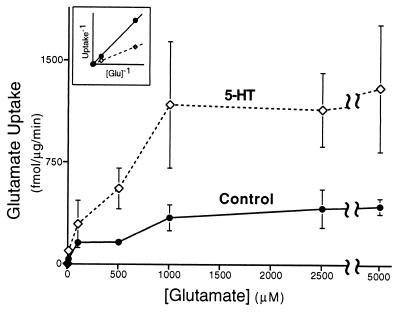
Synaptosomes exhibit high-affinity Glu uptake. Glu uptake was measured in synaptosomal preparations (P3; see Fig. 3A) derived from pleural-pedal ganglia. Synaptosomal Glu uptake was measured from control (solid line, ●) and experimental (500 μM 5-HT, dashed line, ◊) animals 24 h after treatment. Glu uptake by synaptosomes was dose dependent. Note the apparent increase in Vmax from synaptosomes isolated 24 h after treatment of animals with 5-HT. (Inset) Lineweaver–Burk analysis of data in main graph. Each experiment contained synaptosomes derived from 8–10 pleural-pedal ganglia. In this and subsequent illustrations, error bars are SEM.
Results
Characterization of Glu Transporter.
The synaptosomal preparation (P3) from pleural-pedal ganglia exhibited high-affinity Glu uptake (Fig. 1, solid line). Lineweaver–Burk analysis of the observed Glu uptake demonstrated a transporter with Km = 5 μM and Vmax = 255 fmol/μg/min (Fig. 1 Inset), values similar to those measured in other systems (34). Elimination of sodium from the extracellular medium inhibited Glu uptake (10 μM) by 98 ± 5% (SEM, n = 4).
The effects of several known Glu uptake inhibitors were measured. The inhibitors, which are all conformational analogues of Glu, were: THA, pyrrolidine-dicarboxylic acid, and aminocyclobutane-dicarboxylic acid. The relative potencies of these drugs to inhibit uptake into synaptosomes were (IC50): 0.03 mM THA > 0.3 mM pyrrolidine-dicarboxylic acid > 1.0 mM aminocyclobutane-dicarboxylic acid. The efficacy of THA was also tested in intact ganglia, with an estimated IC50 of 6 mM. Several reasons may account for the difference in IC50s for THA measured in synaptosomes and whole ganglia. The connective tissue sheath of the ganglia may present a diffusional barrier. In addition, Glu uptake by whole ganglia is via two different populations of transporters as assessed by Lineweaver–Burk analysis (data not shown), which may influence the ability of the drug to inhibit Glu uptake.
Regulation of Glu Transporters.
To study the regulation of Glu uptake, animals were exposed to one of two different treatments that enhance the siphon withdrawal reflex. The first treatment examined was an in vivo treatment of 5-HT, which produces long-term facilitation. Elimination of 5-HT blocks induction of long-term sensitization (35, 36). In vivo treatment of Aplysia with 5-HT elicits molecular changes associated with long-term sensitization and facilitation (37–39), and elevates the concentration of 5-HT in the hemolymph to μM levels (40) which are known to cause facilitation of the sensorimotor synapse in vitro. Bathing an animal with 500 μM 5-HT for 1.5 h produced a 140% increase in Glu uptake 24 h after treatment (n = 5) and a much smaller increase immediately after the treatment (n = 4) (Fig. 2, F = 3.9, df = 22, P < 0.02). Treatment with 250 μM 5-HT in vivo also increased Glu uptake, but the increase in Glu uptake 24 h after 250 μM 5-HT was much less than the increase produced by 500 μM 5-HT (Fig. 2; P < 0.05). In a set of parallel control experiments, the effect of in vivo 5-HT treatments was investigated on duration of siphon withdrawal; 500 μM 5-HT significantly increased the duration of siphon withdrawal 112 ± 25% 24 h after treatment (F = 12.2, df = 11, P < 0.002), but 250 μM 5-HT did not appear to significantly change the duration of the reflex response (32 ± 9%). Thus, the long-term effects of the two different concentrations of 5-HT on Glu uptake correlated with their effects on behavior.
Long-term sensitization of the siphon withdrawal reflex is classically produced by electrical stimulation applied to one side of the body wall (6, 11). Sensitization training led to a significant long-term (24 h) increase (120%) in Glu uptake in synaptosomes obtained from pleural-pedal ganglia from the trained side of the animal compared with control synaptosomes obtained from contralateral ganglia on the untrained side of the animal (Fig. 2; n = 5, P < 0.05). We confirmed in parallel experiments that electrical stimulation produced unilateral sensitization (Fig. 5). Thus, two different treatments (5-HT and electrical stimulation) which induced a long-term enhancement of the siphon-withdrawal reflex significantly increased Glu uptake in synaptosomes.
Site of Transporter Regulation.
To investigate whether the increase in Glu uptake was caused by glial or neuronal uptake (34), we compared the relative abundance of glia and nerve terminals with the effects of 5-HT on Glu uptake in two different fractions generated by the synaptosomal isolation technique (Fig. 3A). Immunoblots for the Aplysia glial-specific protein Ag were performed (31) to determine the presence of glia and immunoblots for synaptophysin were used to assess the presence of nerve terminals (30). Densitometric analysis indicated that Ag was 10-fold enriched in P2 (cellular fraction pellet) relative to P3 (synaptosomal fraction pellet) (Fig. 3B, n = 3). In contrast, synaptophysin was threefold enriched in P3 relative to P2 (Fig. 3B, n = 4).
If the increase in Glu uptake after 5-HT was caused by an increase in glial uptake, then the increase in Glu uptake should be larger in the P2 fraction than the P3 preparation. Conversely, if the increase in Glu uptake was caused by neuronal uptake, then the increase in Glu uptake should be larger in the P3 than the P2 preparation. As previously observed, Glu uptake was significantly increased in P3 (n = 8), the synaptosomal preparation, 24 h after treatment with 5-HT (Fig. 3C, t = 5.9, df = 11, P < 0.0002). However, no change in Glu uptake 24 h after treatment with 5-HT was observed in P2 (n = 5), the cellular preparation which contained the most glia (Fig. 3C). The absence of any effect in the cellular fraction was surprising, because the cellular fraction has been shown to contain more total synaptosomes than the synaptosomal fraction (29). The specific activity of Glu uptake in the cellular fraction is double that of the synaptosomal fraction (cellular, 520 fmol/μg/min; and synaptosomal, 237 fmol/μg/min). However, as indicated by our Western blots and other studies (29), glial and other nonsynaptic membrane is highly abundant in the cellular fraction. Therefore, absence of an effect in the cellular fraction is most likely caused by a masking effect of glial uptake. Because the synaptosomal fraction has little glial membrane and is enriched in presynaptic membrane (Fig. 3B), the increase in Glu uptake characterized in synaptosomes obtained from treated animals was most likely caused by an increase in neuronal, rather than glial Glu uptake. It is possible that the cellular compartments which were purified by synaptosomal isolation technique contained glial or other cellular elements which did not express Ag or synaptophysin. Therefore, we used cultures of isolated sensory neurons to investigate whether 5-HT could elicit an increase in Glu uptake specifically in sensory neurons.
The effect of 5-HT on Glu uptake was measured in individual cultures of sensory neurons. No other cell types were present in the cultures. Sensory neurons exhibited sodium-dependent Glu uptake (data not shown). Treatment of the cultures with 50 μM 5-HT for 1.5 h produced a significant 130% increase in Glu uptake 24 h after the treatment (Fig. 3C; df = 3, P < 0.05). Thus, 5-HT can produce an increase in Glu uptake in sensory neurons.
Specificity of Transporter Regulation.
The increase observed in synaptosomal Glu uptake after exposure to 5-HT or sensitization training could be caused by an increase in sodium-dependent or sodium-independent uptake. We found that Glu uptake in the absence of extracellular sodium was unaffected by in vivo 5-HT treatments (Fig. 4A; t = 1.31, df = 5, P < 0.25). Therefore, long-term increases in Glu uptake produced by 5-HT were caused by sodium-dependent uptake mechanisms.
To investigate the possibility that transport of amino acids other than Glu was regulated, uptake of Leu and Gln was examined. Leu serves as a marker for general amino acid uptake because it is not transported via a high-affinity transporter. Leu uptake was unaffected by treatments in vivo with 5-HT (Fig. 4A; t = 0.50, df = 3, P < 0.65).
In the central nervous system, glia take up Glu and convert it to Gln via glutamine synthetase, an enzyme which is also found in Aplysia glia (41). Gln is then released by glia and taken up by neurons as a precursor for synthesis of Glu or as an energy source. We found that Gln uptake was significantly increased 150% by treatment with 5-HT (Fig. 4A; t = 3.25, df = 5, P < 0.05). Regulation of Gln transport appeared to be neuronal because the effect of 5-HT on Gln uptake was observed only in the P3 preparation (see above) and not in the P2 preparation (P2 = −20.38 ± 10.53%, t = 1.94, df = 2, P < 0.20). The increase in synaptosomal Gln uptake could be caused by the increase in activity of the Glu transporter. However, Gln uptake was not inhibited by molar excess Glu (Fig. 4B). In addition, Glu uptake was not inhibited by molar excess Gln (Fig. 4C). These results indicate that Glu and Gln transporters are different entities.
Mechanism of Regulation.
To determine whether the Km or Vmax of the Glu transporter was affected by 5-HT, animals were treated with 5-HT and the kinetics of Glu uptake from synaptosomes were measured. The Vmax of Glu uptake was significantly increased approximately threefold 24 h after treatment with 500 μM 5-HT (795 fmol/μg/min, Fig. 1, dashed line; F = 3.72, df = 90, P < 0.05). However, the Km for Glu uptake (6 μM) was not significantly different from control values (5 μM) (t = 0.53, df = 90, P < 0.8).
Increases in Vmax could be caused by insertion of transporters into the plasma membrane which were cytosolic before induction of long-term sensitization (42), or newly synthesized transporters could be added to the membrane via movement through the Golgi and transport to the nerve terminal (43–45). The time course of the increase in Glu uptake suggested synthesis of new proteins and their subsequent transport to the nerve terminal (Fig. 2). To test this hypothesis, animals were injected with emetine (1 ml, 9 mM/140 g body weight), a protein synthesis inhibitor, or DRB (3 ml, 2 mM/100 g body weight), a mRNA synthesis inhibitor (46), before receiving long-term sensitization training. The experimentor who measured the duration of siphon withdrawal was blind to the side that received electrical stimulation and the solution which was injected into the animal. Injection of emetine into an intact Aplysia has previously been shown to inhibit protein synthesis within 0.5 h by greater than 90% in pleural-pedal ganglia (40). Injection of DRB inhibited RNA synthesis by 50 ± 7% (SEM, n = 3) within 0.5 h as assayed by incorporation of uridine into trichloroacetic acid-precipitable material (46). Long-term sensitization training increased by 130% the duration of siphon withdrawal elicited from the side of the animal which received the training (Fig. 5), but not the contralateral side (5 ± 3%, n = 5). Injection of animals with emetine or DRB 30 min before electrical stimulation significantly inhibited sensitization of the siphon withdrawal reflex (Fig. 5; df = 10, P < 0.05). Duration of the siphon withdrawal elicited from the untreated side of the animal was not affected by emetine (6 ± 3%, n = 3) or DRB (14 ± 20%). The large increase in Glu uptake normally seen 24 h after sensitization training (Fig. 3B, replotted in Fig. 5) was significantly inhibited by injection of emetine (n = 4) or DRB (n = 3) before electrical stimulation (Fig. 5, P < 0.05). Emetine or DRB injection alone did not affect basal Glu uptake [(in fmol/μg/min) control, 469 ± 84; emetine, 435 ± 29; DRB, 400 ± 40] suggesting that synaptic Glu transporters have a relatively slow turnover rate. These results indicate that synthesis of mRNA and protein is required for the increase in Glu uptake. However, because both mRNA and protein synthesis are required for induction of long-term facilitation, the requirement for protein and mRNA synthesis may occur at earlier steps and not the synthesis of new transporters.
To further investigate the possible role of Glu transporter synthesis, isolated pleural-pedal ganglia were exposed to 5-HT and then brefeldin A, a specific inhibitor of endoplasmic reticulum-Golgi transfer (47) which has no effect on delivery of glucose transporters to the plasma membrane by exocytosis (48). Treatment of isolated pleural-pedal ganglia with 50 μM 5-HT induced an increase in synaptosomal Glu uptake 24 h after treatment (Fig. 6; F = 4.17, df = 23, P < 0.01). Treatment of pleural-pedal ganglia with brefeldin A inhibited the increase in Glu uptake caused by 5-HT (Fig. 6, n = 5, P < 0.05). Treatment with brefeldin A alone did not change Glu uptake, providing further evidence that synaptic Glu transporters have a slow turnover rate (Fig. 6, n = 5). Together, the results obtained with emetine and brefeldin A suggest that long-term regulation of Glu transporters may involve synthesis of new transporters and their transfer through the Golgi. It should be noted that brefeldin A has been shown to inhibit long-term potentiation in area CA1 of the hippocampus 3 h after induction (49); therefore, brefeldin A may inhibit the increase in Glu uptake by blocking an induction event involved in the pathway responsible for long-term regulation of Glu uptake. Further work is required to distinguish between the possible mechanisms of action of DRB, emetine, and brefeldin A on long-term regulation of Glu uptake.
Discussion
Treatments that induce long-term facilitation or behavioral sensitization increased neuronal Glu uptake. This increase in Glu uptake occurred in sensory neurons and appears to be caused by an increase in the number of Glu transporters. An increase in the number of transporters could be caused by insertion of additional transporters at existing sensorimotor synapses (42–45), an increase in the size or number of synapses (7, 8), or a combination of these possibilities. First, 1 day of sensitization training, which is what was used in our experiments, does not induce significant morphological change in sensory neurons of the abdominal (7, 8) or pleural-pedal ganglia (50). Morphological change has only been observed after 4 days of sensitization training (7, 8, 50). Second, in our synaptosomal preparation, results are normalized for protein, potentially removing any increase that may be caused by addition of new terminals. However, the small amount of protein new terminals would contribute and the heterogeneity of the synaptosomal preparation (29) make it difficult to exclude the possibility that growth of new synapses partially contributes to the increase in synaptosomal Glu uptake. Finally, although application of 5-HT does not increase the number of varicosities in isolated sensory neurons (10), we observed changes in uptake in isolated sensory neurons. It should be noted that the concentration of 5-HT used in the aforementioned study was 10 times lower than the concentration used in our study. Thus, it is possible that growth might account for a portion of the increase in Glu uptake we observed in neuronal cultures. Altogether, it seems as if the increase in Glu uptake and the number of transporters in the membrane we observed was not caused by growth of new terminals. However, we cannot rule out that growth may partially account for the increase in Glu uptake.
Termination of neurotransmission at glutamatergic synapses occurs via diffusion and the action of synaptic Glu transporters (20, 21). Long-term facilitation of the sensorimotor synapse of Aplysia is caused, in part, by an increase in the release of neurotransmitter which appears to be Glu (9, 14–19). Increases in Glu uptake concomitant with increases in Glu release may have several important functions. Increasing Glu uptake could prevent extrasynaptic diffusion of Glu from active synapses (51). Diffusion of Glu outside of the synaptic cleft may activate presynaptic metabotropic receptors (52–54) or Glu receptors within neighboring synapses (51). The activity of Glu transporters may also impact the duration of the excitatory postsynaptic potential (19, 55). More importantly, increases in Glu uptake may act to prevent depletion of transmitter and/or desensitization of synaptic Glu receptors (22, 27, 56–62), and may also supply additional Glu for energy metabolism. Thus, Glu transporters may play an important role in maintaining an increased efficacy of transmission at glutamatergic synapses.
The increase in Gln uptake may serve several functions. Increased release of Glu caused by facilitation of neurotransmitter release may require increased production of Glu from Gln to maintain the neurotransmitter pool. Gln also could be used for production of energy via the tricarboxylic acid cycle and may serve as a source of nitrogen for the biosynthesis of several nitrogen-containing compounds. Finally, some studies have implicated Gln in the synthesis of nitric oxide via Arg.¶ NO has been implicated in several examples of neuronal plasticity (63–67).
Regulation of the Glu and Gln transporters by treatments which cause long-term sensitization in Aplysia indicate that neurotransmitter uptake and synthesis may be important for expression of plasticity and therefore be coregulated with transmitter release. In support of this hypothesis, results indicate that regulation of Glu uptake also occurs in area CA1 of the hippocampus during long-term potentiation (68). Thus, regulation of Glu uptake during increases in synaptic efficacy such as long-term facilitation in Aplysia and long-term potentiation in mammals may be a phylogenetically conserved phenomenon. Moreover, these transporters provide new molecular correlates which can now be used to study mechanisms involved in long-term changes related to the formation of memory. Our findings present an opportunity to study the long-term regulation of neurotransmitter transporters because relatively little is known regarding these mechanisms in either long-term memory or other instances such as neurodegenerative disorders (69) or severe brain trauma (70), where regulation of transporters seems to be involved.
Acknowledgments
The authors thank J. Liu, X. Ren, and C. Tehlirian for technical assistance. The Ag antibodies used in this study were a generous gift from Dr. I. Levitan. This work was supported by National Institutes of Health Grants NS28462 to A.E. and NS19895 to J.H.B.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- DRB
5,6-dichlorobenzimidazole riboside
- THA
dl-threo-β-hydroxyaspartate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220256497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220256497
O'Dowd, Y. & Newsholme, P. (1997) Biochem. Soc. Trans. 25, 403S (abstr.).
References
- 1.Byrne J H, Baxter D A, Buonomano D V, Cleary L J, Eskin A, Goldsmith J R, McClendon E, Nazif F A, Noel F, Scholz K P. Ann N Y Acad Sci. 1991;627:124–149. doi: 10.1111/j.1749-6632.1991.tb25918.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C H, Bartsch D, Kandel E R. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel T, Martin K C, Bartsch D, Kandel E R. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 4.Bailey C H, Chen M. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- 5.Dale N, Kandel E R, Schacher S. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz K P, Byrne J H. Science. 1987;235:685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- 7.Bailey C H, Chen M. Proc Natl Acad Sci USA. 1988;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey C H, Chen M. Proc Natl Acad Sci USA. 1988;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale N, Schacher S, Kandel E R. Science. 1988;239:282–285. doi: 10.1126/science.2892269. [DOI] [PubMed] [Google Scholar]
- 10.Glanzman D L, Kandel E R, Schacher S. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- 11.Cleary L J, Lee W L, Byrne J H. J Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montarolo P G, Goelet P, Castellucci V F, Morgan J, Kandel E R, Schacher S. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 13.Bailey C H, Montarolo P, Chen M, Kandel E R, Schacher S. Neuron. 1992;9:749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- 14.Dale N, Kandel E R. Proc Natl Acad Sci USA. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X Y, Glanzman D L. Proc R Soc London B. 1994;255:215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Wu F, Schacher S. J Neurosci. 1997;17:4976–4986. doi: 10.1523/JNEUROSCI.17-13-04976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armitage B A, Siegelbaum S A. J Neurosci. 1998;18:8770–8779. doi: 10.1523/JNEUROSCI.18-21-08770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levenson J, Sherry D M, Dryer L, Chin J, Byrne J H, Eskin A. J Comp Neurol. 2000;423:121–131. doi: 10.1002/1096-9861(20000717)423:1<121::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Burdohan J, Levenson J, Eskin A, Byrne J H. Soc Neurosci Abstr. 1998;24:1701. [Google Scholar]
- 20.Schousboe A. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls D, Attwell D. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 22.Otis T S, Wu Y C, Trussell L O. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hestrin S, Sah P, Nicoll R A. Neuron. 1990;5:247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- 24.Barbour B, Keller B U, Llano I, Marty A. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 25.Tong G, Jahr C E. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 26.Diamond J S, Jahr C E. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turecek R, Trussell L O. J Neurosci. 2000;20:2054–2063. doi: 10.1523/JNEUROSCI.20-05-02054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng K T, O'Dowd B S, Rickard N S, Robinson S R, Gibbs M E, Rainey C, Zhao W Q, Sedman G L, Hertz L. Neurosci Biobehav Rev. 1997;21:45–54. doi: 10.1016/0149-7634(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 29.Chin G J, Shapiro E, Vogel S S, Schwartz J H. J Neurosci. 1989;9:38–48. doi: 10.1523/JNEUROSCI.09-01-00038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin G J, Vogel S S, Elste A M, Schwartz J H. Brain Res. 1990;508:265–272. doi: 10.1016/0006-8993(90)90405-z. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart S T, Levitan I B, Pikielny C W. J Neurobiol. 1996;29:35–48. doi: 10.1002/(SICI)1097-4695(199601)29:1<35::AID-NEU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Schacher S, Proshansky E. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin J, Angers A, Cleary L J, Eskin A, Byrne J H. Learn Mem. 1999;6:317–330. [PMC free article] [PubMed] [Google Scholar]
- 34.Kanai Y, Smith C P, Hediger M A. FASEB J. 1993;7:1450–1459. doi: 10.1096/fasebj.7.15.7903261. [DOI] [PubMed] [Google Scholar]
- 35.Walters E T, Byrne J H, Carew T J, Kandel E R. J Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 36.Glanzman D, Mackey S, Hawkins R, Dyke A, Lloyd P, Kandel E. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberini C M, Ghirardi M, Metz R, Kandel E R. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 38.Hegde A N, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain D G, Martin K C, Kandel E R, Schwartz J H. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 39.Bartsch D, Casadio A, Karl K A, Serodio P, Kandel E R. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 40.Levenson J, Byrne J H, Eskin A. J Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roots B I. J Exp Biol. 1981;95:167–180. doi: 10.1242/jeb.95.1.167. [DOI] [PubMed] [Google Scholar]
- 42.Corey J L, Davidson N, Lester H A, Brecha N, Quick M W. J Biol Chem. 1994;269:14759–14767. [PubMed] [Google Scholar]
- 43.Ramamoorthy S, Cool D R, Mahesh V B, Leibach F H, Melikian H E, Blakely R D, Ganapathy V. J Biol Chem. 1993;268:21626–21631. [PubMed] [Google Scholar]
- 44.Ramamoorthy J D, Ramamoorthy S, Papapetropoulos A, Catravas J D, Leibach F H, Ganapathy V. J Biol Chem. 1995;270:17189–17195. doi: 10.1074/jbc.270.29.17189. [DOI] [PubMed] [Google Scholar]
- 45.Rattray M, Wotherspoon G, Savery D, Baldessari S, Marden C, Priestley J V, Bendotti C. Eur J Pharmacol. 1994;268:439–442. doi: 10.1016/0922-4106(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 46.Raju U, Koumenis C, Nunez-Regueiro M, Eskin A. Science. 1991;253:673–675. doi: 10.1126/science.1871602. [DOI] [PubMed] [Google Scholar]
- 47.Chardin P, McCormick F. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 48.Bao S, Smith R M, Jarett L, Garvey W T. J Biol Chem. 1995;270:30199–30204. doi: 10.1074/jbc.270.50.30199. [DOI] [PubMed] [Google Scholar]
- 49.Matthies H, Jr, Kretlow J, Matthies H, Smalla K H, Staak S, Krug M. Neuroscience. 1999;91:175–183. doi: 10.1016/s0306-4522(98)00628-9. [DOI] [PubMed] [Google Scholar]
- 50.Wainwright M L, Zhang H, Byrne J H, Cleary L J. Soc Neurosci Abstr. 1997;23:1334. [Google Scholar]
- 51.Asztely F, Erdemli G, Kullmann D M. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 52.Cochilla A J, Alford S. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 53.Dube G R, Marshall K C. J Neurophysiol. 2000;83:1141–1149. doi: 10.1152/jn.2000.83.3.1141. [DOI] [PubMed] [Google Scholar]
- 54.Abdul-Ghani A S, Attwell P J, Singh Kent N, Bradford H F, Croucher M J, Jane D E. Brain Res. 1997;755:202–212. doi: 10.1016/s0006-8993(97)00098-x. [DOI] [PubMed] [Google Scholar]
- 55.Mennerick S, Zorumski C F. Nature (London) 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 56.Kiskin N I, Krishtal O A, Tsyndrenko A. Neurosci Lett. 1986;63:225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- 57.Dudel J, Franke C, Hatt H, Ramsey R L, Usherwood P N. Neurosci Lett. 1988;88:33–38. doi: 10.1016/0304-3940(88)90311-4. [DOI] [PubMed] [Google Scholar]
- 58.Trussell L O, Thio L L, Zorumski C F, Fischbach G D. Proc Natl Acad Sci USA. 1988;85:4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang C M, Dichter M, Morad M. Science. 1989;243:1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- 60.Trussell L O, Fischbach G D. Neuron. 1989;3:209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- 61.Raman I M, Trussell L O. Biophys J. 1995;68:137–146. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otis T, Zhang S, Trussell L O. J Neurosci. 1996;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller U. Neuron. 1996;16:541–549. doi: 10.1016/s0896-6273(00)80073-2. [DOI] [PubMed] [Google Scholar]
- 64.Kendrick K M, Guevara-Guzman R, Zorrilla J, Hinton M R, Broad K D, Mimmack M, Ohkura S. Nature (London) 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- 65.Rickard N S, Ng K T, Gibbs M E. Neurobiol Learn Mem. 1998;69:79–86. doi: 10.1006/nlme.1997.3806. [DOI] [PubMed] [Google Scholar]
- 66.Lewin M R, Walters E T. Nat Neurosci. 1999;2:18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- 67.Zhuo M, Laitinen J T, Li X C, Hawkins R D. Learn Mem. 1999;6:63–76. [PMC free article] [PubMed] [Google Scholar]
- 68.Levenson J, Weeber E, Selcher J, Kategaya L, Sweatt J, Eskin A. Soc Neurosci Abstr. 2000;26:1590. [Google Scholar]
- 69.Rothstein J D, Van Kammen M, Levey A I, Martin L J, Kuncl R W. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 70.Rossi D J, Oshima T, Attwell D. Nature (London) 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]