Abstract
Cortical maps express experience-dependent plasticity. However, the underlying cellular mechanisms remain unclear. We have recently shown that sensory deprivation results in large changes of the short-term dynamics of excitatory synapses at the junction of deprived and spared somatosensory (barrel) cortex, which may contribute to map reorganization. A key issue is whether the alterations in short-term synaptic dynamics are driven by a loss of sensory input or by competition between deprived and spared inputs. Here, we report that short-term dynamics of horizontal pathways in the middle of uniformly deprived cortex change only modestly. Vertical intracortical pathways were unaffected by deprivation. Our results suggest that uniform loss of sensory activity has a limited effect on short-term synaptic dynamics. We concluded that competition between sensory inputs is necessary to produce large-scale changes in synaptic dynamics after sensory deprivation.
Experience-dependent plasticity of cortical maps occurs in somatosensory (1), visual (2), and auditory (3) sensory systems. Competition between sensory inputs has been shown to play an important role, particularly in the visual cortex (4, 5). The effects of competition are easiest to study during developmental “critical periods” when map reorganization tends to be greatest. However, it is now clear that plasticity of many cortical maps persists into adulthood (6, 7), and experiments suggest the possibility that competition between sensory inputs continues to be involved in cortical map reorganization of older animals (8).
Whereas competition may manifest itself at the level of cortical maps, the synaptic correlates of competition remain unclear. Competition between sensory inputs over a time scale of hours has been studied in the retino-tectal system, where it is suggested that long-term potentiation (LTP) and long-term depression (LTD) do play roles in map development (9). However, it has been difficult to prove or refute the involvement of LTP and LTD in reorganization of cortical maps because of technical problems in measuring LTP/LTD at the same synapses over the duration of sensory modification protocols, which typically last days.
Investigation in vitro of the cellular mechanisms underpinning competition is further confounded by the fact that the most common method of inducing competition involves altering sensory input. Thus, it is difficult to study competition in isolation. The information contained within the short-term synaptic dynamics offered a possible solution. We have recently shown that brief periods of innocuous sensory deprivation result in large-amplitude changes of the short-term dynamics of horizontal excitatory synapses at the junction of deprived and spared cortex, and we have suggested how these changes could contribute to map reorganization (10). The key issue that arises from these experiments is whether alterations in short-term synaptic dynamics are driven by a loss of sensory input per se or by competition between deprived and spared inputs. We reasoned that we could tease apart the contribution of a loss of sensory input from the effect of competition between deprived and spared sensory inputs by comparing the synaptic dynamics in the middle of deprived cortex with those in the middle of spared cortex. The results could then be contrasted with the synaptic dynamics at the junction of deprived and spared cortex (10), and the relative contributions of loss of sensory input and competition between sensory inputs could be assessed.
Materials and Methods
Trimming Protocol.
Rats had all of the whiskers on either the right or left side of the snout trimmed flush with the fur every day for 5 or 10–14 days. The rats were gently restrained during the trimming. Median ages of 5-day deprivation (24.5 days, interquartile range 19–28 days) and 10- to 14-day deprivation rats (25 days, interquartile range 24–26 days) on the experimental (i.e., terminal) day were not different (P = 0.85, Mann–Whitney rank sum test). The advantage of this trimming protocol is that it allows comparison of deprived and spared cortex in the same animal. One possible concern is that there may still be some residual sensory input to deprived cortex via the transcallosal pathway that links homotopic points in somatosensory cortex. However, the transcallosal pathway is weak (11–13), and its effect appears to be primarily inhibitory (14). Furthermore, lesioning the transcallosal input results in short-lasting (<30 min) alterations of receptive fields with no evidence of long-term plasticity (15).
Slice Preparation and Recordings.
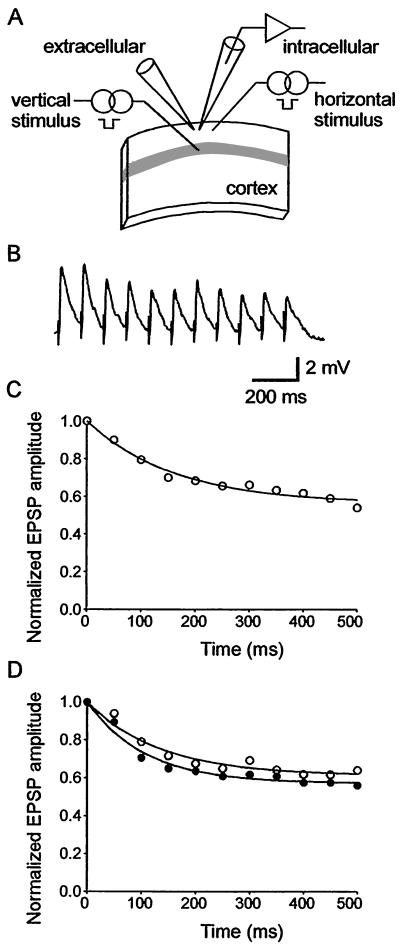
On the day of recording, rats had all whiskers trimmed acutely by an assistant to blind the electrophysiologist to the rat's sensory history. Thalamocortical brain slices were prepared from both hemispheres as described previously (16) and stored in an interface chamber at the junction between artificial cerebrospinal fluid (ACSF) flowing at 1 ml/min and humidified 95%/5% O2/CO2. The ACSF contained (in mM): NaCl 124, KCl 3, NaH2PO4 1.25, NaHCO3 26, dextrose 10, MgCl2 1, and CaCl2 2, and was saturated with 95%/5% O2/CO2. The bath temperature was 34°C. Angled illumination allowed identification of the upper border of the barrels (lower layer 3). Ultrasmall concentric bipolar stimulation electrodes (25-μm tip; FHC, Bowdoinham, ME) were located in layer 4 and layer 2/3 (Fig. 1A). A large bore micropipette (0.5–1 MΩ) filled with 0.2 mM bicuculline methiodide dissolved in ACSF was located in layer 2/3 to block fast inhibitory postsynaptic potentials focally. We recorded excitatory postsynaptic potentials (EPSPs) from neurons in the immediate vicinity of the field potential electrode with sharp microelectrodes (70–100 MΩ) filled with 3 M potassium acetate and 1% biocytin. Pyramidal cells were identified electrophysiologically on the basis of their firing characteristics. A baseline monosynaptic EPSP of approximately 5–7 mV was evoked. We ensured that fast inhibitory postsynaptic potentials were blocked by stimulating vertical and horizontal intracortical afferents while depolarizing layer 2/3 neurons with current pulses and checking for the presence of a hyperpolarizing potential overlapping the EPSP (17). N-methyl-D-aspartate (NMDA) receptors were blocked with 50 μM DL-2-amino-5-phosphopentanoic acid (AP5, Sigma) in the ACSF. EPSPs were evoked by stimulus trains comprising eleven stimuli at a constant frequency. The results were averaged over three trials, with each trial separated by 30 s. This protocol was repeated at stimulation frequencies varying from 5–40 Hz. In each animal, we recorded from at least one neuron in both spared and deprived hemispheres. When recordings from one neuron were completed, we moved stimulating and recording electrodes to another slice from the opposite hemisphere and recorded from the homotopic point. Synaptic responses were recorded with an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) and digitized at 10 KHz. Data acquisition and signal analysis were done in the LABVIEW environment (National Instruments, Austin TX).
Figure 1.
Short-term synaptic dynamics in the thalamocortical slice. (A) Schematic diagram of the position of the recording and stimulating electrodes positions in primary somatosensory cortex. The gray band represents layer 4 of the barrel field. A field electrode was used to apply bicuculline methiodide focally. (B) Average responses (3 trials) evoked by stimulation of the horizontal layer 2–2 pathway at 20 Hz in one slice from deprived cortex. (C) Single exponential fit to the responses in B. (D) Single exponential fit to the responses evoked by 20-Hz stimulus trains averaged across all recordings in deprived cortex (open circles, n = 14 neurons from 10 slices) and spared cortex (filled circles, n = 12 neurons from 10 slices) of rats trimmed for 5 days.
Data Analysis.
Single exponential curve fitting was done using the Marquardt–Levenburg algorithm (18). When calculating the time constant of EPSP amplitude depression, we required that there were at least two points on the rapidly decaying part of the fitted curve to ensure that the fit reflected the synaptic dynamics. Thus, we had to exclude the 5-Hz data from the analysis of synaptic strength because EPSP amplitudes frequently reached a steady state by the second response in the train. Inter-animal variability is a potential cause of bias. Therefore, if we recorded more than one neuron from a hemisphere, we averaged the results for that hemisphere so that, for each animal, we had one set of results for the deprived cortex paired with results obtained in nondeprived cortex. The variance of the time constant of EPSP amplitude decay data decreased with the mean as stimulation frequency increased. Hence, parametric statistical tests could not be used on the raw data. Where appropriate, we have used a logarithmic transform to equalize the variances (19) before proceeding to paired t tests. Alternatively, we used nonparametric statistical analysis on the raw data. Details of the model have been described elsewhere (10, 20).
Results
We induced an innocuous but extensive sensory deprivation of primary somatosensory cortex (SI) by trimming all whiskers from one side of rats' snouts for 5 days (10 rats, 5 right trim and 5 left trim) or 10–14 days (7 rats, 3 right trim and 4 left trim). Another group of rats, handled in the same way, but without trimming, acted as a sham-control (4 rats). We prepared in vitro thalamocortical slices (16) from both hemispheres and made intracellular recordings from layer 2/3 pyramidal neurons in the whisker barrel part of primary somatosensory cortex (Fig. 1A).
Neither deprivation status nor duration of deprivation affected intrinsic membrane properties of pyramidal neurons (Table 1). We evoked EPSPs by stimulating either the horizontal layer 2 to layer 2 pathway or the vertical layer 4 to layer 2 pathway with trains of stimuli at frequencies from 5–40 Hz (n = 26 neurons after 5-day deprivation, n = 19 neurons after 10-day deprivation, and n = 9 neurons in sham-controls; Fig. 1B). N-methyl-D-aspartate (NMDA) receptor-mediated excitatory potentials and fast γ-aminobutyric acid receptor-mediated inhibitory potentials were blocked pharmacologically. We measured the amplitude of EPSPs during the stimulus trains and normalized the amplitudes to the first response in the train. Single exponentials were fitted to the results to estimate the normalized steady-state amplitude and the time constant of EPSP amplitude depression. This analysis was done for each slice (Fig. 1C) and for data pooled into deprived and spared groups (Fig. 1D).
Table 1.
Intrinsic properties of layer 2/3 pyramidal cells
| Deprived | Spared | Pooled control | |
|---|---|---|---|
| 5-day deprivation | |||
| Resting membrane potential, mV | −79 ± 1 | −79 ± 2 | −80 ± 1 |
| Input resistance, MΩ | 36 ± 5 | 33 ± 4 | 34 ± 2 |
| Membrane time constant, ms | 11 ± 1 | 12 ± 2 | 12 ± 1 |
| 10-day deprivation | |||
| Resting membrane potential, mV | −80 ± 1 | −80 ± 1 | −80 ± 1 |
| Input resistance, MΩ | 37 ± 3 | 37 ± 4 | 34 ± 2 |
| Membrane time constant, ms | 13 ± 1 | 13 ± 1 | 12 ± 1 |
All data are given as mean ± SEM. Two-way ANOVA showed no difference in resting membrane potential (n = 42; deprivation status, F1,39 = 0.01, P = 0.91; trim duration, F1,36 = 0.84, P = 0.37), input resistance (n = 42; deprivation status, F1,39 = 0.23, P = 0.64; trim duration; F1,39 = 0.38, P = 0.54) or membrane time constant (n = 39; deprivation status, F1,36 = 0.59, P = 0.45; trim duration, F1,36 = 1.83, P = 0.19) when deprived and spared cortex following 5-day deprivation or 10–14 day deprivation were compared. One-way ANOVA showed no difference in resting membrane potential (5-day trim, F2,34 = 0.23, P = 0.80; 10-day trim, F2,25 = 0.02, P = 0.98), input resistance (5-day trim, H = 0.66, P = 0.72; 10-day trim, F2,25 = 0.20, P = 0.83) or membrane time constant (5-day trim, F2,31 = 0.67, P = 0.52; 10-day trim, F2,25 = 0.53, P = 0.59) when deprived and spared cortex were compared with pooled controls.
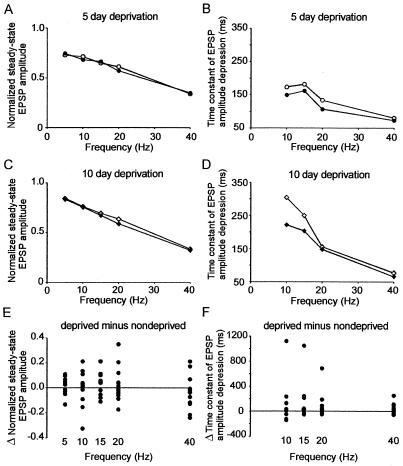
We found that there were subtle changes in the short-term dynamics of excitatory synapses in the horizontal 2–2 pathway after 5 days of deprivation. Although there was no statistically significant difference in the normalized steady state amplitude of horizontal 2–2 pathways in supragranular cortex (5- to 40-Hz data, mean difference = 0.006 ± 0.108; P = 0.73, paired t test, n = 39; Fig. 2A), the time constant of EPSP amplitude depression was longer in the deprived hemisphere at all frequencies tested (10- to 40-Hz data, mean difference = 33 ms, 95% confidence interval 2–72 ms; P = 0.04, paired t test, n = 29; Fig. 2B). We trimmed a second group of rats for 10–14 days to determine whether longer periods of deprivation produced greater changes in short-term synaptic dynamics. There was no consistent difference in the normalized steady-state amplitude of horizontally evoked EPSPs in deprived and spared cortex (5- to 40-Hz data, mean difference = 0.046 ± 0.032; P = 0.16, paired t test, n = 24; Fig. 2C). However, the time constant of EPSP amplitude depression was longer in the deprived hemisphere compared with the spared hemisphere at all frequencies tested (10- to 40-Hz data, median difference = 19.5 ms; P = 0.17, Wilcoxon signed rank test, n = 19)(Fig. 2D). We pooled the results from both studies to see whether there were consistent changes in short-term synaptic dynamics. There was no difference in normalized steady-state (Fig. 2E), but the time constant of EPSP decay was shorter in nondeprived rats (median difference = 25 ms; P = 0.02, Wilcoxon signed rank test, n = 48; Fig. 2F). There was no significant correlation between the steady-state amplitude and time constant of EPSP amplitude decay (r = −0.02; P = 0.86, Pearson product moment test, n = 96). This result suggests that the rate of decrease of EPSP amplitude during a stimulus train and the steady-state amplitude could be modulated separately.
Figure 2.
Horizontal layer 2 to layer 2 pathway. (A) Normalized steady-state EPSP amplitudes evoked by stimulation of layer 2–2 pathways in deprived (open circles, n = 10) or spared cortex (filled circles, n = 10) with brief stimulus trains of varying frequencies after 5 days of deprivation. (B) Time constant of EPSP amplitude depression calculated from single exponential fits to normalized EPSP amplitudes evoked by stimulation of layer 2–2 pathways in deprived (open circles, n = 10) or spared cortex (filled circles, n = 10) at varying frequencies after 5 days of deprivation. (C) Normalized steady-state EPSP amplitudes evoked by stimulation of layer 2–2 pathways in deprived (open circles, n = 7) or spared cortex (filled circles, n = 7) with brief stimulus trains of varying frequencies after 10–14 days of deprivation. (D) Time constant of EPSP amplitude depression calculated from single exponential fits to normalized EPSP amplitudes evoked by stimulation of layer 2–2 pathways in deprived (open circles, n = 7) or spared cortex (filled circles, n = 7) at varying frequencies after 10–14 days of deprivation. (E) Pooled differences in normalized steady state after 5- and 10-day trims. (F) Pooled differences in time constant of EPSP amplitude depression after 5- and 10-day trims.
We analyzed our data in more detail by using a model of short-term synaptic dynamics (10, 20). The key benefit of the model is that it describes the varying EPSP amplitudes during a stimulus train, which we refer to as the synaptic efficacy, with parameters that remain constant during that train. Values for the parameters can be used to compare quantitatively differences between deprived and spared cortex or between different trimming protocols. Characterization of normalized EPSP amplitudes during a stimulus train requires only two parameters. One parameter represents the proportion of synaptic resources, S, used to generate an EPSP with each action potential. This parameter, which varies between zero and one, can be thought of as the synaptic strength and, for simplicity, that is how we refer to it. The second parameter, τ, denotes the time constant with which synaptic resources recover after having been used to generate an EPSP. The model predicts that synaptic efficacy depresses exponentially toward a steady state during a stimulus train. Therefore, we used the single exponential fits to calculate values for synaptic strength and the time constant of recovery at varying frequencies for deprived and spared hemispheres.
We found that there was a small but consistent decrease in predicted synaptic strength of horizontal layer 2–2 pathways in deprived cortex compared with spared cortex after 5 days (10- to 40-Hz data, deprived = 0.17 ± 0.03, spared = 0.20 ± 0.03; P = 0.065, paired t test) and 10–14 days of deprivation (10- to 40-Hz data, deprived = 0.12 ± 0.03, spared = 0.15 ± 0.03; P = 0.004, paired t test). The time constant of recovery decreased by a small amount after 5 days of deprivation (10- to 40-Hz data, deprived = 270 ± 10 ms, spared = 240 ± 10 ms; P = 0.004, paired t test), but it was not statistically different from that in spared cortex after 10–14 days of deprivation (10- to 40-Hz data, deprived = 330 ± 40 ms, spared = 280 ± 20 ms; P = 0.18, paired t test). Results from spared cortex were not different from pooled controls [synaptic strengths, spared cortex (pooled 5- and 10-day deprivation) = 0.18 ± 0.02, control = 0.16 ± 0.03, P = 0.45; time constant of recovery, spared cortex = 260 ± 20 ms, controls 270 ± 40 ms, P = 0.89]. Taken together, the results suggested that 5 or 10–14 days of sensory deprivation induced a modest, but highly statistically significant weakening of synaptic strength in deprived cortex compared with spared cortex (pooled 5 and 10–14 days deprivation data, P < 0.001). Moreover, our data indicated that the change in synaptic strength had developed by 5 days of deprivation and did not tend to become much larger with more prolonged deprivation. Our experiments do not give a lower limit on how fast changes in the short-term dynamics of excitatory synapses develop. A whisker trimming protocol that leaves two neighboring whiskers results in changes in receptive fields of those whiskers within 3 days of trimming in adult rats (21, 22). Thus, it is clearly possible that short-term synaptic dynamics change rapidly after trimming, but a longitudinal study is required to resolve this issue.
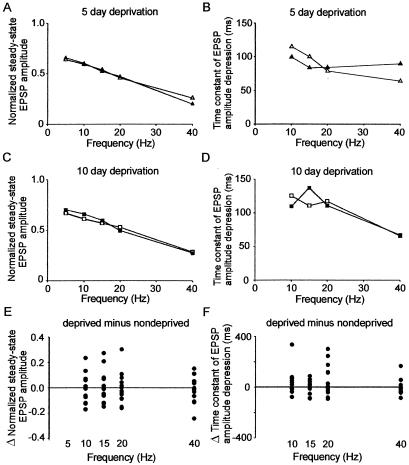
We undertook a similar analysis of short-term synaptic dynamics of the vertical layer 4 to layer 2 pathway in deprived and spared hemispheres to assess whether they were modified by the trimming protocol. We found that there was no difference between the normalized steady-state amplitudes in deprived and spared cortex after 5 days (5- to 40-Hz data, deprived minus spared = 0.007 ± 0.015; P = 0.67; Fig. 3A) or 10–14 days of deprivation (5- to 40-Hz data, deprived minus spared = −0.014 ± 0.016; P = 0.43; Fig. 3C). Similarly, time constants of EPSP amplitude depression of vertical layer 4–2 pathways were the same in deprived and spared cortex after 5 days (10- to 40-Hz data, deprived minus spared = 0.2 ± 9.9 ms; P = 0.98; Fig. 3B) and 10–14 days of deprivation (10- to 40-Hz data, deprived minus spared = −1.5 ± 9.1 ms; P = 0.88, paired t test; Fig. 3D). By using the model, analysis of the results from the layer 4 to layer 2 pathway showed that sensory deprivation did not alter synaptic strength after 5 days (10- to 40-Hz data, deprived = 0.31 ± 0.02, spared = 0.32 ± 0.04; P = 0.82) or 10–14 days of sensory deprivation (10- to 40-Hz data, deprived = 0.26 ± 0.03, spared = 0.25 ± 0.03; P = 0.75). Similarly, the time constant of recovery in deprived cortex was not different from that in spared cortex after 5 days (10- to 40-Hz data, deprived = 240 ± 20 ms, spared = 280 ± 70 ms; P = 0.53) or 10–14 days of deprivation (10- to 40-Hz data, deprived = 250 ± 10 ms, spared = 250 ± 20 ms; P = 0.92). Results from the trimmed rats were not different from pooled controls [synaptic strengths, spared cortex (pooled 5- and 10-day deprivation) = 0.29 ± 0.03, control = 0.28 ± 0.03, P = 0.77; time constant of recovery, spared cortex = 260 ± 40 ms, controls 310 ± 30 ms, P = 0.07]. We concluded that the short-term synaptic dynamics of the vertical layer 4-layer 2 pathway in the middle of deprived cortex were not modified by sensory deprivation.
Figure 3.
Vertical layer 4 to layer 2 pathway. (A) Normalized steady-state EPSP amplitudes evoked by stimulation of layer 4–2 pathways in deprived (open circles, n = 10) or spared cortex (filled circles, n = 10) with brief stimulus trains of varying frequencies after 5 days of deprivation. (B) Time constant of EPSP amplitude depression calculated from single exponential fits to normalized EPSP amplitudes evoked by stimulation of layer 4–2 pathways in deprived (open circles, n = 10) or spared cortex (filled circles, n = 10) at varying frequencies after 5 days of deprivation. (C) Normalized steady-state EPSP amplitudes evoked by stimulation of layer 4–2 pathways in deprived (open circles, n = 7) or spared cortex (filled circles, n = 7) with brief stimulus trains of varying frequencies after 10–14 days of deprivation. (D) Time constant of EPSP amplitude depression calculated from single exponential fits to normalized EPSP amplitudes evoked by stimulation of layer 4–2 pathways in deprived (open circles, n = 7) or spared cortex (filled circles, n = 7) at varying frequencies after 10–14 days of deprivation. (E) Pooled differences in normalized steady state after 5- and 10-day trims. (F) Pooled differences in time constant of EPSP amplitude depression after 5- and 10-day trims.
Discussion
Our experiments show that a modest change in synaptic strength and dynamics of the horizontal pathways in the middle of deprived cortex had developed after 5 days of deprivation. Furthermore, these changes did not tend to become much larger with more prolonged deprivation. The results from our current experiments bear similarities to the results that we found at the junction of deprived and spared cortex (10). Greater changes in short-term synaptic dynamics occur in the horizontal layer 2 to layer 2 pathways compared with the vertical layer 4 to layer 2 pathway. Furthermore, the pattern of the results found in the horizontal layer 2–2 pathway (altered synaptic strength, unchanged time constant of recovery) was the same as the results found at the junction of deprived and spared cortex after 10 days of deprivation.
However, we noted one big difference when we compared results from the middle of deprived cortex with those from the edge of deprived cortex. The changes in synaptic dynamics were much larger at the junction of deprived and spared cortex. This finding can be quantified by using the model. The magnitude of the difference between the synaptic strength in spared and deprived cortex in the current experiments was only 0.03 (19% of control synaptic strength) at 10 days. This result was much less than the difference in synaptic strength between horizontal inputs onto deprived cortex at the junction of deprived and spared cortex, which was 0.13 (48% of control synaptic strength) (10). Our analysis suggests that the effects of sensory deprivation in the middle of deprived cortex are smaller than those at the edge of deprived cortex. Furthermore, our results indicate that loss of sensory input is not sufficient to account for the changes we saw at the junction of deprived and spared cortex. We conclude that the difference in synaptic strength of horizontal layer 2 to layer 2 excitatory synapses onto deprived cortex at the junction of deprived and spared cortex was the result of a combination of loss of sensory input and competition between sensory inputs.
The effects of trimming all whiskers from one side of the snout for 7 days have been studied in vivo. The largest decrease of neuronal firing was in layer 2/3, with a modest reduction of layer 4 firing and no change in thalamic activity (8). Our results are consistent with this study. However, it is unclear whether our findings can account for all of the reduction in layer 2/3 firing, particularly because the changes we found occur during the early part of stimulus trains and have minimal effect on normalized steady-state EPSP amplitudes. Alterations in inhibitory intracortical circuitry may also be present. Alternatively, changes in subcortical inputs may be important (23). For instance, a reduction in the strength of thalamocortical pathways could account for the reduced layer 4 firing. The reduction in layer 2/3 firing may then be a combination of reduced excitatory inputs from layer 4 and, as we report here, weakened horizontal intracortical inputs from other layer 2/3 pyramidal neurons. Clearly, further experiments are required to resolve the relative contributions of thalamocortical and intracortical inputs to cortical map reorganization in somatosensory cortex.
Evidence suggestive of competition between sensory inputs has been found in primary somatosensory cortex at the junction of deprived and spared cortex (8). However, competition has been most clearly documented in the visual cortex where monocular deprivation during the critical period results in rearrangement of the ocular dominance map (4, 24). Typically, experiments involve recording neuronal firing in vivo in response to a sensory stimulus. Complementary theoretical studies (25–28) attempt to explain competition at a synaptic level. The general thesis is that a change in sensory input manifests itself as an altered pattern of neuronal firing, which in turn leads to activity-dependent modification of synapses. Theories have tended to focus on LTP and LTD or their equivalents as candidate cellular mechanisms. Not only has this work deepened our understanding by describing the constraints on LTP/LTD that are required to fully explain competition (29), but it has also revealed the conflicting ways that LTP and LTD could account for the experimental findings (25, 26, 29). Assessing which theory is most accurate is a pressing problem. However, it has been difficult to perform crucial experiments to test predictions from different theories because of the lack of a simple measure of the average amount or degree of potentiation at synapses.
Neocortical brain slices in vitro have been used to test more general predictions of the theoretical models that relate to experience-dependent plasticity rather than to specifically address competition issues. Results from some experiments suggest that LTP and LTD are involved in experience-dependent plasticity (30, 31). However, it has been questioned whether conventional extracellular stimulation protocols used to generate LTP/LTD in neocortical brain slices in vitro produce the same type of plasticity that occurs when cortical maps reorganize in vivo (32, 33). It is possible that long-lasting LTP, which occurs over days, is different from LTP that is generated in vitro and typically studied over the following hours (34). Global mechanisms, which regulate synaptic efficacy up or down to keep it within meaningful ranges, have been recently described in neurons cultured from visual cortex (35). However, these mechanisms affect all synapses and, therefore, do not deal directly with competition between sensory inputs.
Synaptic transmission in neocortex is a dynamic process (10, 36, 37). We have exploited this fact to gain further insights into the mechanisms of cortical map reorganization in supragranular layers of primary somatosensory cortex, where cortical maps are plastic into adulthood (38–41). Our experiments indicate that loss of sensory input does not account for all of the changes in short-term synaptic dynamics that follow sensory deprivation, and that competition between sensory inputs contributes to cortical map reorganization. Furthermore, our findings suggest the possibility that competition between sensory inputs occurs continuously, but manifests itself only when altered sensory input leads to cortical map reorganization. Thus, we propose that, where cortex remains plastic, there is competition for cortical space.
Acknowledgments
We thank Langdon Roberts for assistance with the preparation of the rats. This work was supported by a Wellcome Trust Advanced Training Fellowship to G.T.F., and a National Institutes of Health grant (NS25983) to B.W.C.
Abbreviations
- EPSP
excitatory postsynaptic potential
- IPSP
inhibitory postsynaptic potential
- LTP
long-term potentiation
- LTD
long-term depression
- ACSF
artificial cerebrospinal fluid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230175697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230175697
References
- 1.Merzenich M M, Kaas J H, Wall J, Nelson R J, Sur M, Felleman D. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 2.Kaas J H, Krubitzer L A, Chino Y M, Langston A L, Polley E H, Blair N. Science. 1990;248:229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- 3.Robertson D, Irvine D. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 4.Wiesel T N, Hubel D H. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 5.Stryker M P, Harris W A. J Neuroscience. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert C D. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 8.Glazewski S, McKenna M, Jacquin M, Fox K. Eur J Neurosci. 1998;10:2107–2116. doi: 10.1046/j.1460-9568.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L I, Tao H W, Holt C E, Harris W A, Poo M-M. Nature (London) 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 10.Finnerty G T, Roberts L S E, Connors B W. Nature (London) 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- 11.Wise S P, Jones E G. J Comp Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- 12.Koralek K A, Olavarria J, Killackey H P. J Comp Neurol. 1990;299:133–150. doi: 10.1002/cne.902990202. [DOI] [PubMed] [Google Scholar]
- 13.Pidoux B, Verley R. Electroencephalogr Clin Neurophysiol. 1979;46:715–726. doi: 10.1016/0013-4694(79)90111-1. [DOI] [PubMed] [Google Scholar]
- 14.Calford M B, Tweedale R. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- 15.Clarey J C, Tweedale R, Calford M B. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- 16.Agmon A, Connors B W. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 17.Connors B W, Malenka R C, Silva L R. J Physiol (London) 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press W H, Flannery B P, Teukolsky B P, Vetterling W T. Numerical Recipes. Cambridge, U.K.: Cambridge Univ. Press; 1986. [Google Scholar]
- 19.Snedecor G W, Cochran W G. Statistical Methods. 6th Ed. Ames, IA: Iowa State Univ. Press; 1967. [Google Scholar]
- 20.Tsodyks M V, Markram H. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond M E, Armstrong-James M, Ebner F F. Proc Natl Acad Sci USA. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong-James M, Diamond M E, Ebner F F. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolelis M A L, Katz D, Krupa D J. Rev Neurosci. 1998;9:213–224. doi: 10.1515/revneuro.1998.9.3.213. [DOI] [PubMed] [Google Scholar]
- 24.Wiesel T N, Hubel D H. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 25.Stent G S. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bienenstock E L, Cooper L N, Munro P W. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von der Malsburg C. Kybernetik. 1973;14:85–100. doi: 10.1007/BF00288907. [DOI] [PubMed] [Google Scholar]
- 28.Miller K D, Keller J B, Stryker M P. Science. 1989;245:605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- 29.Miller K D. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood A, Lee H K, Bear M F. Nature (London) 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood A, Rioult M C, Bear M F. Nature (London) 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 32.Hensch T K, Stryker M P. Science. 1996;272:554–557. doi: 10.1126/science.272.5261.554. [DOI] [PubMed] [Google Scholar]
- 33.Hensch T K, Gordon J A, Brandon E P, McKnight G S, Idzerda R L, Stryker M P. J Neurosci. 1998;18:2108–2117. doi: 10.1523/JNEUROSCI.18-06-02108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens C F. Neuron. 1998;20:1–2. doi: 10.1016/s0896-6273(00)80426-2. [DOI] [PubMed] [Google Scholar]
- 35.Turrigiano G G, Leslie K R, Desai N S, Rutherford L, Nelson S B. Nature (London) 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 36.Markram H, Tsodyks M. Nature (London) 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 37.Abbot L F, Varela J A, Sen K, Nelson S B. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 38.Fox K. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox K. J Neurosci. 1994;14:7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond M E, Huang W, Ebner F F. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- 41.Glazewski S, Fox K. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]