Abstract
Peptidoglycan recognition proteins (PGRPs) are highly conserved pattern-recognition molecules of the innate immune system that bind bacterial peptidoglycans (PGNs), which are polymers of alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) cross-linked by short peptide stems. Human PRGPs are bactericidal against pathogenic and nonpathogenic Gram-positive bacteria, but not normal flora bacteria. Like certain glycopeptide antibiotics (e.g., vancomycin), PGRPs kill bacteria by directly interacting with their cell wall PGN, thereby interfering with PGN maturation. To better understand the bactericidal mechanism of PGRPs, we determined the crystal structure of the C-terminal PGN-binding domain of human PGRP-Iβ in complex with NAG-NAM-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala, a synthetic glycopeptide comprising a complete PGN repeat. This structure, in conjunction with the previously reported NMR structure of a dimeric PGN fragment, permitted identification of major conformational differences between free and PGRP-bound PGN with respect to the relative orientation of saccharide and peptide moieties. These differences provided structural insights into the bactericidal mechanism of human PGRPs. On the basis of molecular modeling, we propose that these proteins disrupt cell wall maturation not only by sterically encumbering access of biosynthetic enzymes to the nascent PGN chains, but also by locking PGN into a conformation that prevents formation of cross-links between peptide stems in the growing cell wall.
Keywords: bacteria, cell wall, crystal structure, innate immunity
The innate immune system is a host defense mechanism, evolutionarily conserved from insects to mammals, which mediates early recognition and control of invading microorganisms (1, 2). It recognizes microbes by means of pattern recognition molecules, such as Toll-like receptors and collectins, which bind unique products of microbial metabolism not produced by the host (pathogen-associated molecular patterns). Examples include lipopolysaccharide, mannans, nonmethylated CpG sequences, and peptidoglycan (PGN) (1, 2).
Peptidoglycan recognition proteins (PGRPs) are pattern-recognition molecules that bind and, in certain cases, hydrolyze PGNs of bacterial cell walls (3, 4). PGRPs are found in both invertebrates and vertebrates, but have developed different functions in different animals. Insect PGRPs are involved in the Toll receptor and Imd-signaling pathways that induce expression of antimicrobial peptides (2, 5, 6). By contrast, mammalian PGRPs do not act through host signaling pathways but are directly bactericidal (5–10). Human PGRP-Iα and -Iβ are secreted proteins with strong bactericidal activity against pathogenic and nonpathogenic Gram-positive bacteria, but not normal flora bacteria (10). They are selectively expressed in tissues exposed to the environment, including the oral cavity, intestinal tract, and skin (5). Human PGRP-S, found in polymorphonuclear leukocyte granules, is directly bactericidal for both Gram-positive and -negative bacteria (7, 8, 10). Whereas PGRP-Iα and -Iβ each comprise two tandem PGN-binding domains, PGRP-S consists of a single such domain (5, 6). Mammalian PGRPs kill 99% of bacteria at 0.1- to 1-μM concentrations and are therefore more potent, on a molar basis, than most antimicrobial peptides (10).
Unlike mammalian antimicrobial peptides such as defensins (11, 12), which permeabilize bacterial membranes, human PGRPs kill bacteria by interacting with their cell walls, where they are believed to interfere with PGN biosynthesis (10). In this respect, PGRPs function like glycopeptide antibiotics, including vancomycin and teicoplanin, which inhibit PGN synthesis by binding PGN or its precursors (13, 14). As cross-linked PGN provides the mechanical support necessary to prevent bacterial cells from rupturing as osmotic pressure fluctuates, interfering with cross-linking provides an effective means for disrupting the structural integrity of bacteria (13, 14).
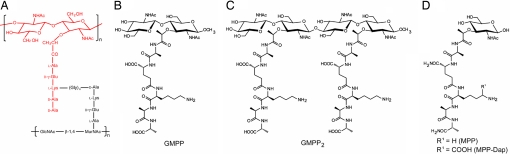
PGNs are polymers of alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) in β (1–4) linkage, cross-linked by short peptide stems composed of alternating l- and d-amino acids (Fig. 1A) (3, 4). Transglycosylases catalyze polymerization of lipid II to generate the polymeric PGN unit, which is cross-linked to an existing PGN unit in the cell wall by transpeptidases (13, 14). Whereas the carbohydrate backbone is conserved in all bacteria, the peptide displays diversity. According to the residue at position 3 of the stems, PGNs are divided into two categories: l-lysine type (Lys-type) and diaminopimelate type (Dap-type). Dap-type PGN stems are usually directly cross-linked, whereas Lys-type PGN stems are interconnected by a peptide bridge that varies in length and composition in different bacteria (Fig. 1A).
Fig. 1.
Structure of PGN and PGN derivatives. (A) Schematic representation of Lys-type PGNs. Lys-type PGN peptides are usually cross-linked through a peptide bridge composed of one to five glycines. The fragment shown in red corresponds to GMPP. (B) Chemical structure of GMPP. (C) GMPP2. (D) MPP (R1, H) and MPP-Dap (R1, COOH).
Vancomycin and related glycopeptide antibiotics form a noncovalent complex with the d-Ala-d-Ala portion of cell wall PGN, resulting in steric inhibition of the transglycosylation and/or transpeptidation steps of PGN synthesis (13, 14). Although human PGRPs appear to act through a similar mechanism (10), x-ray crystallographic studies have shown that these proteins bind both the glycan and peptide portions of PGNs (6, 15), as exemplified by the structure of the C-terminal PGN-binding domain of human PGRP-Iα (PGRP-IαC) bound to a muramyl pentapeptide (MPP), NAM-l-Ala-γ-d-Gln-l-Lys-d-Ala-d-Ala (16). However, because MPP lacks the NAG moiety present in all PGNs, the PGRP-IαC–MPP complex only partially defined the interaction with the carbohydrate backbone of PGN. To better understand how mammalian PGRPs recognize native PGN, we determined the crystal structure of the C-terminal PGN-binding domain of human PGRP-Iβ (PGRP-IβC) in complex with NAG-NAM-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala, a glucosamyl MPP (GMPP) containing a complete disaccharide repeat (Fig. 1B). This structure, combined with the recent NMR structure of a dimeric PGN fragment (Fig. 1C), NAG-NAM(-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala)-NAG-NAM(-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala) (GMPP2) (17), enabled us to identify conformational changes in PGN induced by PGRP binding. These interactions suggest a mechanism whereby PGRPs disrupt cell wall maturation in a manner that is related to, yet distinct from, the mechanism used by glycopeptide antibiotics.
Results and Discussion
PGN Analog Binding to Human PGRPs.
The PGN-binding properties of human PGRP-IβC, as well as those of PGRP-IαC and -S, were characterized by isothermal titration calorimetry (ITC) using Lys-type PGN analogs GMPP, GMPP2, and MPP and the Dap-type analog NAM-l-Ala-γ-d-Gln-Dap-d-Ala-d-Ala (MPP-Dap) (Fig. 1 B–D). ITC revealed exothermic heats of reaction for the binding of PGRP-IβC to GMPP and GMPP2 [supporting information (SI) Fig. 5], with equilibrium binding constants (Kbs) of 2.3 × 105 M−1 and 2.4 × 105 M−1, respectively (Table 1). A stoichiometry of unity was observed for both reactions, indicating that GMPP2, despite being dimeric, could only accommodate a single PGRP. Significantly, vancomycin binds GMPP and GMPP2 with affinities very similar to those of PGRP-IβC for these same ligands, although GMPP2 engages two vancomycins (18). PGRP-IβC and -IαC have similar PGN-binding characteristics, except that PGRP-IαC recognized GMPP and GMPP2 with ≈4-fold lower Kb than PGRP-IβC. PGRP-IβC and -IαC each bound MPP ≈1.5-fold less tightly than GMPP or GMPP2 (SI Fig. 5), indicating only a minor contribution from the NAG moiety present in both GMPP and GMPP2, but not in MPP. Neither PGRP-IβC nor -IαC bound MPP-Dap, even under conditions at which interactions with Kb>100 M−1 should be detectable (19, 20). Thus, both these PGRP domains appear highly specific for Lys-type PGN. By contrast, PGRP-S preferentially recognized MPP-Dap over MPP. In addition, the NAG moiety of GMPP improved affinity ≈14-fold relative to MPP, showing that the extra saccharide contributes substantially more to PGN binding in the case of PGRP-S than of PGRP-IβC or -IαC (Table 1). Notably, all three PGRPs bound GMPP2, the ligand most representative of natural PGN, with comparable Kbs.
Table 1.
Binding constants (×103 M−1) of PGN derivatives to human PGRPs at 275–277 K
| Protein | GMPP | GMPP2 | MPP | MPP-Dap |
|---|---|---|---|---|
| PGRP-IβC | 227 (±14) | 235 (±10) | 118 (±4) | NB |
| PGRP-IαC | 59 (±2) | 61 (±2) | 45 (±1)* | NB* |
| PGRP-S | 82 (±3) | 131 (±7) | 6 (±0)* | 47 (±6)* |
For all PGN derivatives, n values ranged from 0.98 to 1.07. Values in parentheses represent uncertainties of fit. NB, no binding detectable.
*Binding data are from ref. 20.
Overview of the Structure.
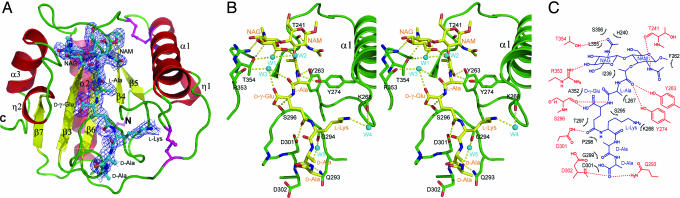
The structures of PGRP-IβC in free form and bound to GMPP were determined to resolutions of 2.2 and 2.1 Å, respectively (SI Table 3). The asymmetric unit of the PGRP-IβC-GMPP crystal contains three PGRP-IβC molecules; however, only one forms a complex with GMPP because the binding sites of the other two are blocked by neighboring PGRP-IβC molecules. Obvious electron density corresponding to the entire GMPP ligand was found in the binding cleft, as evident from 2Fo–Fc and Fo–Fc electron density maps (Fig. 2A). After refinement, the site occupancy was set to 1.0 and the average temperature factor (B) was 38.4 Å2, compared with 29.6 Å2 for main-chain atoms (SI Table 3). Superposition of unbound PGRP-IβC domains from both structures onto PGRP-IβC–GMPP gave rms differences in α-carbon positions of 0.23–0.48 Å, indicating no substantial conformational changes upon binding. PGRP-IβC shows a typical PGN-binding domain scaffold (6), in which six β-strands (β3–7) compose a central β-sheet surrounded by three α-helices (α1–3) and two short 310 helices (η1 and η2) (Fig. 2A). Three disulfide bonds cross-link the protein.
Fig. 2.
Structure of the PGRP-IβC–GMPP complex. (A) Overall structure. Disulfide bonds are shown in purple. Labeling of secondary structure elements follows the numbering for unbound PGRP-IαC in ref. 21. The N and C termini are indicated. The bound GMPP is shown in ball-and-stick representation, with carbon atoms in cyan, nitrogen atoms in blue, and oxygen atoms in red. The final σA-weighted 2Fo–Fc electron density map for the GMPP ligand at 1.5 σ is contoured in blue. (B) Stereoview of interactions between PGRP-IβC and GMPP at the PGN-binding site. The bound GMPP is shown in stick representation, with carbon atoms in yellow, nitrogen atoms in blue, and oxygen atoms in red. Hydrogen bonds are drawn as dotted lines. Bound waters (W1–5) mediating hydrogen bonds between PGRP-IβC and GMPP are shown as cyan balls. (C) Schematic representation of interactions between PGRP-IβC and GMPP. GMPP is shown in blue; hydrogen bonds are shown as dotted lines. Residues making van der Waals contacts with GMPP are indicated by arcs with spokes radiating toward the ligand moieties they contact. Water-mediated interactions between GMPP and PGRP-IβC are omitted for clarity.
Characteristics of the PGN-Binding Cleft.
The PGN-binding site of PGRP-IβC, whose general topology is maintained in other PGRPs (15, 21–28), resides in a long cleft whose walls are formed by helix α1 and five loops (β3–α1, 1α–β4, β5–β6, β6–α2, β7–α3) that project above the central β-sheet platform (Fig. 2 A and B). The groove is ≈27 Å long, with a shallow (≈6 Å) end flanked by helix α1 and loops β3–α1 and β6–α2 and a deep (≈12 Å) end flanked by loops 1α–β4, β5–β6, and β7–α3. There are two main structural differences between the PGN-binding sites of PGRP-IβC and -IαC, which differ in 22 of 41 residues lining the grooves (SI Fig. 6A). First, loops β5–β6 and β7–α3, which form one wall, provide a broader surface for accommodating NAG in the PGRP-IβC-GMPP complex. Second, the C-terminal portion of helix α1 is displaced away from the PGN-binding site in PGRP-IβC compared with its position PGRP-IαC, further widening the cleft (SI Fig. 6 B and C).
Interactions in the PGN-Binding Site.
In the PGRP-IβC-GMPP structure, the pentapeptide stem of the ligand is held in an extended conformation at the deep end of the binding groove, and the NAG-NAM disaccharide fills a pocket in the middle (Fig. 2B). The unoccupied shallow end is predicted to accommodate a repeating NAG-NAM unit of polymeric PGN (see below). GMPP is 55% buried in the PGN-binding site, where it makes extensive interactions with 21 residues lining the binding cleft through 17 hydrogen bonds, of which 7 are water-mediated, and 90 van der Waals contacts (Fig. 2 B and C). Two of three hydrogen bonds directly linking PGRP-IβC to NAM (Thr-241 O–N2 NAM and Tyr-274 Oη–O10 NAM) are conserved in the PGRP-IαC-MPP structure, as are all three hydrogen bonds between PGRP-IβC and γ-d-Glu-2 (SI Table 4). However, PGRP-IβC and -IαC differ significantly in their interactions with the two C-terminal residues of peptide stem (d-Ala-4-d-Ala-5), which adopt different conformations in the corresponding complexes. Thus, PGRP-IβC does not contact d-Ala-4, whereas PGRP-IαC engages this residue through both direct and solvent-mediated hydrogen bonds (16). Conversely, PGRP-IαC makes only a few contacts with d-Ala-5, whereas PGRP-IβC interacts extensively with this residue.
In contrast to PGRP-S, PRGP-IβC, like PGRP-IαC, has no detectable affinity for the Dap-type PGN analog MPP-Dap (Table 1). Structure-based sequence alignments with Drosophila PGRP-LE, which preferentially recognizes Dap-type PGNs, revealed that PGRP-IβC lacks a critical Arg at position 288 (Val in both PGRP-IβC and -IαC), but that PGRP-S retains an Arg at this position. In the structure of PGRP-LE bound to a Dap-type PGN ligand (27), Arg-254 makes a bidentate salt bridge with the side-chain carboxylate of Dap. The corresponding Val-288 of PGRP-IβC, unlike Arg-88 of PGRP-S, could not form this presumably stabilizing interaction.
Conformational Differences in Free Versus PGRP-Bound PGN Derivatives.
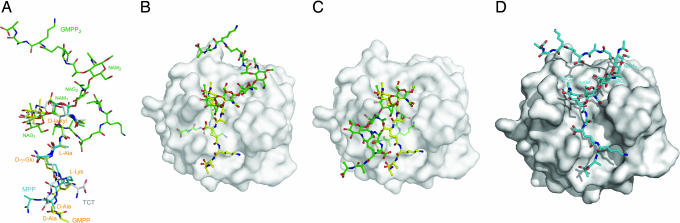
Crystal structures of PGN fragments bound to human or insect PGRPs (15, 16, 27, 28), including the PGRP-IβC–GMPP complex, have shown that the fragments adopt a similar overall conformation and interaction mode, with some variations around the saccharide and C terminus of the peptide stem (Fig. 3A). Thus, NAM and the N-terminal portion (d-lactyl-l-Ala-d-γ-Glu) of the pentapeptide stem of GMPP superpose well onto the corresponding parts of MPP (16) and tracheal cytotoxin (TCT) (27, 28), a monomeric fragment of Dap-type PGN, even though TCT contains an anhydro form of NAM [NAG-NAM(1,6-anhydro)-l-Ala-d-γ-Gln-Dap-d-Ala].
Fig. 3.
Structural comparison between PGRP-bound PGN analogs in crystal structures and unbound GMPP2 in solution. (A) Conformational comparison of GMPP, MPP, TCT, and GMPP2. GMPP, MPP, and TCT are from crystal structures of complexes with human PGRP-IβC, human PGRP-IαC (16), and Drosophila PGRP-LE (27), respectively; GMPP2 is from the unliganded NMR structure (17). The structures are superposed through the pyranose ring of NAM (for MPP, GMPP, and GMPP2) or NAM(1,6-anhydro) (for TCT). (B) Superposition of unbound GMPP2 onto GMPP in the PGRP-IβC–GMPP complex. GMPP and GMPP2 are shown in ball-and-stick representations, with carbon atoms in yellow and green, respectively, nitrogen atoms in blue, and oxygen atoms in red. Of the two GMPP units in GMPP2, the first unit, comprising the NAG1-NAM1 disaccharide, is superposed onto GMPP in the complex. The peptide stem of GMPP2 attached to NAM1 is buried within PGRP-IβC and is shown in pale green. (C) Alternative superposition of unliganded GMPP2 onto GMPP bound to PGRP-IβC. In this case, the second GMPP unit of GMPP2, containing NAG2-NAM2, is superposed onto GMPP in the PGRP-IβC–GMPP structure. The peptide stem of GMPP2 attached to NAM2, shown in pale green, is buried inside PGRP-IβC. (D) Modeled PGRP-IβC–GMPP2 structure.
The NMR structure of an unbound PGN monomer [NAG-NAM-l-Ala-d-γ-Gln-Dap-d-Ala-d-Ala] has been reported (29). More recently, the solution structure of unbound GMPP2 (Fig. 1C) was determined (Fig. 3A), and a model of the bacterial cell wall on the basis of this PGN dimer was proposed (17). In both structures, the glycan portion and d-lactyl moiety connecting NAM to the pentapeptide stems exist in well-defined conformations, whereas the stems display greater mobility. The two dihedral angles between NAG1 and NAM1 in both structures are similar, resulting in almost the same glycan conforma-tion: C2(NAG1)–C1(NAG1)–O1(NAG1)–C4(NAM1) and C1(NAG1)–O1(NAG1)–C4(NAM1)–C3(NAM1) are 174° and 118°, respectively, in the PGN monomer (29), and 173° and 126°, respectively, in GMPP2 (17) (Table 2). Surprisingly, however, the d-lactyl conformation is markedly different between the PGN monomer and dimer structures. The methyl group of the d-lactyl moiety of GMPP2 is close to NAG1 to avoid steric hindrance between NAG1 and pentapeptide stem, with dihedral angles of 61° and −91° for N2(NAM1)–C2(NAM1)–C3(NAM1)–O3(NAM1) and C4(NAM1)–C3(NAM1)–O3(NAM1)–C9(d-lactyl), respectively, which define the relative position of the d-lactyl and tetrasaccharide portions (Table 2). By contrast, in the PGN monomer, these angles are 66° and −159°, respectively. Accordingly, PGN fragments in solution are characterized by a single well defined conformation for the glycan, two distinct conformations for the d-lactyl moiety, a limited number of conformations for the two N-terminal residues of peptide stem, and flexible C termini.
Table 2.
Comparison of selected dihedral angles
| Dihedral angles | PGRP-Iβ C–GMPP | GMPP2 | PGN monomer |
|---|---|---|---|
| NAG-NAM-φ | 163 | 173 | 174 |
| NAG-NAM-ψ | 161 | 126 | 118 |
| d-Lac-ψ | 67 | 61 | 66 |
| d-Lac-φ | −157 | −91 | −159 |
Definitions for dihedral angles are as follows: NAG-NAM-φ, C2(NAG1)–C1(NAG1)–O1(NAG1)–C4(NAM1); NAG-NAM-ψ, C1(NAG1)–O1(NAG1)–C4(NAM1)–C3(NAM1); d-Lac-ψ, N2(NAM2)–C2(NAM2)–C3(NAM2)–O3(NAM2); and d-Lac-φ, C4(NAM2)–C3(NAM2)–O3(NAM2)–C9(d-lactyl). GMPP2 data are for the NMR structure of GMPP2 (17). PGN monomer data are for the NMR structure of Dap-type PGN monomer (NAG-NAM-l-Ala-γ -d-Gln-Dap-d-Ala-d-Ala) (29).
Comparison of these unbound NMR structures with crystal structures of PGRP-bound PGN analogs revealed two main differences (Fig. 3A). In the two solution structures, the conformation of the glycan portion is nearly identical (see above). However, the dihedral angles between NAG1 and NAM1 of GMPP in the PGRP-IβC-GMPP complex are 163° for C2(NAG1)–C1(NAG1)–O1(NAG1)–C4(NAM1) and 161° for C1(NAG1)–O1(NAG1)–C4(NAM1)–C3(NAM1) (Table 2), which are higher in energy than those in the unbound NMR structures (174° and 118°, respectively, for the PGN monomer) on the basis of relaxed conformational maps of the disaccharide 3-Me-4-β-NAG-α-NAM (29). Therefore, it appears that the glycan is distorted upon complex formation with PGRP-IβC (and other PGRPs). As discussed above, there exist at least two stable minima for the conformation of the d-lactyl moiety. In the PGRP-IβC–GMPP structure, the conformation of the d-lactyl moiety of GMPP is similar to that of the PGN monomer (29), with dihedral angles of 67° and −157° for N2(NAM1)–C2(NAM1)–C3(NAM1)–O3(NAM1) and C4(NAM1)–C3(NAM1)–O3(NAM1)–C9(d-lactyl), respectively, which differ markedly from those of GMPP2 (61° and −91°) (Fig. 3A). Other PGRP-bound PGN derivatives (NAM-l-Ala-γ-d-Gln-l-Lys, MPP, TCT) also exhibit this conformation for the d-lactyl moiety (15, 16, 27, 28). Thus, it appears that PGRPs consistently favor one of two possible conformations for this residue.
The reason for this preference is evident from superpositions of unbound GMPP2 (17) onto GMPP in the PGRP-IβC–GMPP complex. In Fig. 3B, GMPP2 is superposed onto GMPP through the first disaccharide (NAG1-NAM1) of the PGN dimer. However, the peptide stem of GMPP2 attached to NAM1 collides with PGRP-IβC, requiring the d-lactyl moiety to adopt the conformation observed in unbound PGN monomer (29). Alternatively, GMPP2 may be superposed onto GMPP through the second disaccharide (NAG2-NAM2) (Fig. 3C). In this case, the peptide stem of GMPP2 attached to NAM2 clashes with the protein, again necessitating a change in orientation relative to the glycan. Importantly, as discussed below, the PGRP-bound conformation is inconsistent with PGN cross-linking as proposed in a model of the cell wall that is based on the GMPP2 structure (17).
Interactions with Polymeric PGN.
PGRP-IβC, -IαC, and -S have comparable affinities for GMPP and GMPP2 (Table 1), suggesting that saccharides beyond NAG1-NAM1 do not contribute substantially to the interaction of PGRPs with PGN. Because we were unable to crystallize PGRP-IβC bound to GMPP2, we modeled the complex on the basis of the PGRP-IβC-GMPP crystal structure, the GMPP2 solution structure (17), and ITC measurements demonstrating that PGRP-IβC, -IαC, and -S all engage GMPP2 with 1:1 stoichiometry.
In the modeled PGRP-IβC-GMPP2 structure (Fig. 3D), the N-acetylamido, hydroxyl, and hydroxymethyl groups of NAG2, along with NAM1 and NAM2, restrict the β (1–4) glycosidic dihedral angles between NAM1 and NAG2. The resulting angles of C2(NAM1)–C1(NAM1)–O1(NAM1)–C4(NAG2) = 172° and C1(NAM1)–O1(NAM1)–C4(NAG2)–C3(NAG2) = 113° are close to those from the NMR structure (173° and 126°, respectively). The β (1–4) glycosidic dihedral angles between NAG2 and NAM2 are also well restricted by the N-acetylamido, methoxyl, and hydroxymethyl groups of NAM2, along with the pentapeptide stem, resulting in angles of 172° and 123°. Under this conformation of NAG2 and NAM2, only NAG2 can interact with PGRP-IβC. In the modeled complex (Fig. 3D), the dihedral angles defining the conformation of the d-lactyl moiety attached to NAM2 are 59° and −89° for N2(NAM2)–C2(NAM2)–C3(NAM2)–O3(NAM2) and C4(NAM2)–C3(NAM2)–O3(NAM2)–C9(d-lactyl), respectively, very similar to those of GMPP2 in solution (61° and −91°) (17). These angles must differ from those defining the relative orientation of d-lactyl and NAM1 in GMPP2 bound to PGRP-IβC (66° and −159°) to avoid major collisions between PGRP-IβC and the peptide stem. In this way, the stem projects away from the protein, with which it makes no contacts (Fig. 3D). Because only NAG2 of the NAG2-NAM2-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala repeat of GMPP2 is predicted to interact with PGRP-IβC, the modeled complex is consistent with our finding that the affinity of GMPP2 for PGRP-IβC is no greater than that of GMPP (Table 1). The binding of two PGRP molecules to GMPP2 would be precluded by steric clashes between the proteins, as well as by the imposition of two distinct conformations on the peptide stems of GMPP2 upon engagement of a single PGRP, only one of which would allow entry of the stem into the PGN-binding groove (Fig. 3B). By contrast, GMPP2 engages two vancomycins, which are considerably smaller than PGRPs and which interact only with the peptide stems of PGN (18), rather than with both glycan and peptide components, as do PGRPs.
Mechanistic Implications.
The bacterial cell wall, far from being a static structure, is dynamically turned over during growth (3, 4). In Escherichia coli, for example, ≈40% of cell wall components are degraded and recycled per generation (30). Furthermore, the degree of PGN cross-linking varies widely for different bacteria (5–75%) (3, 4). As a consequence, there exists an abundance of free peptide stems available as binding sites for bactericidal agents such as PGRPs.
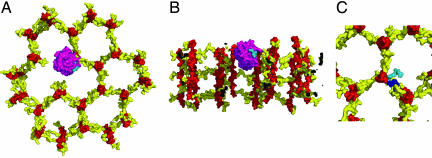
Our results with the PGRP-IβC–GMPP complex may be interpreted in terms of a recent model of cell wall PGN generated in silico by using the GMPP2 solution structure (17). In this model, the glycan strands of PGN are oriented perpendicular to the bacterial cell surface, in sharp contrast to an alternative model that proposes a parallel arrangement (31). A key feature of the perpendicular model is that the PGN strands, whose average length is nine NAG-NAM repeats in E. coli (32), form right-handed helices having three NAG-NAM repeats per turn, such that each strand is positioned for cross-linking up to three neighboring PGN strands (17). The result is a honeycomb pattern with pore sizes determined by the extent of cross-linking (Fig. 4 A and B). The smallest pores, containing three cross-links, are ≈70 Å in diameter, whereas missing strands create pores measuring ≈120 Å (for a single missing strand) or greater (for multiple missing strands). Importantly, all pores with missing strands are sufficiently large to permit a PGN-binding domain, which measures ≈40 Å, to access and bind to the growing cell wall, despite its relatively large size (Fig. 4A). It is also likely that 120-Å pores, formed by a single missing strand, can accommodate two tandem PGN-binding domains (as found in full-length PGRP-Iβ), while pores created by two or more missing strands would certainly be able to do so (data not shown). In addition, a growing cell wall should be fully accessible to any PGRP at its outermost edges.
Fig. 4.
Possible interaction of PGRPs with the bacterial cell wall. (A) Top view of a structural model of a PGRP-IβC molecule bound to cell wall PGN. PGRP-IβC and the cell wall are shown in molecular surface representation with the glycan strands of PGN in red, the peptide stems in yellow, PGRP-IβC in purple, and the PGRP-bound peptide stem in cyan. This model was constructed by docking PGRP-IβC onto a GMPP unit of a perpendicular model of the cell wall (17) in which the PGN strands are orthogonal to the cell membrane. The strands form a honeycomb pattern, with pore sizes determined by the extent of cross-linking. Small pores are formed by cross-linking each PGN strand to three neighboring strands. The PGRP-IβC molecule is situated in an incompletely cross-linked region of the growing cell well where a missing PGN strand creates a larger pore. (B) Side view of the model in A, in which the cell wall has been cut away to expose the bound PGRP protein. (C) Comparison of cross-linked and PGRP-bound peptide stems. A cross-linked peptide stem in the model of cell wall PGN is shown in blue. The same peptide stem, but in its PGRP-bound conformation, is shown in cyan.
These observations, combined with the demonstration that human PGRPs kill Gram-positive bacteria by directly interacting with their cell wall PGN (10), suggest a mechanism for the bactericidal activity of PGRPs reminiscent of that of vancomycin and related glycopeptide antibiotics. In this view, PGRPs disrupt cell wall formation by binding the NAG-NAM-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala repeat of Lys-type PGN, thereby sterically encumbering access of biosynthetic enzymes to the nascent PGN chains. The net result is inhibition of transglycosylase-catalyzed glycan lengthening and/or transpeptidase-catalyzed cross-linking of peptide stems, leaving the cell susceptible to osmotic lysis. Indeed, PGRP-mediated killing of Staphylococcus aureus is prevented in medium containing 0.75 M sucrose (10). Although we have interpreted our results in terms of the perpendicular model of cell wall PGN (17), similar considerations should apply to the parallel model (30).
Beyond simple steric encumbrance, however, the PGRP-IβC-GMPP structure further suggests that human PGRPs might disrupt PGN synthesis by locking PGN into a conformation that could prevent formation of cross-links between peptide stems in the growing cell wall. Because newly elongated glycan strands are not cross-linked, this portion of the cell wall will be mechanically fragile until transpeptidation has occurred (13, 14). Free PGN (as represented by GMPP2) differs from PGRP-bound PGN with respect to the conformation of the d-lactyl group, which substantially alters the relative orientation of saccharide and peptide moieties (Fig. 3A). In terms of our model of PGRP-IβC bound to the cell wall, the effect of this alteration is to position the PGRP-bound peptide such that it is no longer directed toward the peptide of a neighboring PGN strand, with which it could otherwise potentially form a cross-link (Fig. 4C).
Thus, the PGRP-bound conformation of the d-lactyl moiety of PGN appears incompatible with peptide cross-linking, as envisaged in the perpendicular model of the cell wall built using the GMPP2 structure (17). However, as discussed above, PGN fragments in solution can also adopt a second conformation for the d-lactyl moiety that is of comparable stability to the first (29). It is this second conformation that is exclusively observed in PGRP-PGN complexes (Fig. 3A), implying that PGRPs have evolved to disrupt cell wall formation not only by direct steric inhibition of biosynthetic enzymes, but by favoring an orientation of the peptide stems that reduces their availability for the cross-linking reaction.
Methods
PGN Derivatives.
Procedures for synthesizing PGN analogs NAG-NAM-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala, NAG-NAM(-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala)-NAG-NAM(-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala), NAM-l-Ala-γ-d-Gln-l-Lys-d-Ala-d-Ala, and NAM-l-Ala-γ-d-Gln-Dap-d-Ala-d-Ala have been described in refs. 33–35.
PGRP Production.
Human PGRP-S and -IαC were obtained by in vitro folding from E. coli inclusion bodies as described in refs. 21 and 22. A DNA fragment encoding residues 209–373 of PGRP-Iβ was cloned into pT7–7 (Novagen, San Diego, CA). The protein was expressed as inclusion bodies in E. coli BL21(DE3) cells (Invitrogen, Carlsbad, CA). Inclusion bodies were dissolved in 50 mM Tris·HCl (pH 8.0), 8 M urea, 2 mM EDTA, and 5 mM DTT. Solubilized PGRP-IβC was diluted into 1.0 M arginine, 100 mM Tris·HCl (pH 8.5), 2 mM EDTA, 6.3 mM cysteamine, and 3.7 mM cystamine to 50 μg/ml. After 3 days at 4°C, the folding mixture was dialyzed against 50 mM Tris·HCl (pH 8.5), and the protein was purified using MonoQ and Superdex 75 HR columns (Amersham Biosciences, Piscataway, NJ).
ITC Measurements and Analysis.
Thermodynamic parameters for the binding of PGRPs to PGN derivatives were determined using a MicroCal VP-ITC titration calorimeter as described in ref. 20. For the present titrations, c values (the product of the initial PGRP concentration and Kb) ranged from 3.06 to 7.99, allowing for precise determination of Kb (19, 36).
Crystallization and Data Collection.
Crystals of free PGRP-IβC (10 mg/ml) grew at room temperature in 0.1 M NiSO4 and 15% (wt/vol) PEG-3350. Crystals of the PGRP-IβC–GMPP complex grew in 15% (vol/vol) Tacsimate (Hampton Research, Riverside, CA), 0.1 M Hepes (pH 7.0), and 2% (wt/vol) PEG-3350 from solutions containing a 3-fold molar excess of GMPP. Both crystals were cryoprotected by soaking in reservoir solutions containing 10% (wt/vol) sucrose. Diffraction data were collected in-house at 100 K by using an R-Axis IV++ image plate detector (Rigaku, Tokyo, Japan). The data were processed using d*TREK incorporated in the CrystalClear version 1.35 software suite (Molecular Structure, The Woodlands, TX) (SI Table 3).
Structure Determination and Refinement.
The structure of unliganded PGRP-IβC was solved by molecular replacement with the program Molrep (37). A homology modeled structure of PGRP-IβC (38), on the basis of the structure of PGRP-IαC (21) [Protein Data Bank (PDB) ID code 1SK3], was used as the search probe. Two obvious solutions corresponding to two PGRP-IβC monomers in the asymmetric unit resulted in a correlation coefficient of 0.53 and Rcryst of 48.5% at 30.0–3.0 Å. Refinement was performed using CNS (39). After initial rigid-body refinement, the correlation coefficient was 0.67, and Rcryst was 45.3%. Manual model rebuilding was carried out in XtalView (40) on the basis of σA-weighted 2Fo–Fc and Fo–Fc electron density maps. The final model has Rcryst of 22.9% and Rfree of 28.5% at 2.2-Å resolution (SI Table 3).
The structure of the PGRP-IβC-GMPP complex was determined by molecular replacement using the structure of unliganded PGRP-IβC as the probe. Three PGRP-IβC molecules were unambiguously located in the asymmetric unit with a correlation coefficient of 0.67 and Rcryst of 51.3% at 30.0–3.0 Å. Refinement was carried out as above, but only one GMPP molecule could be identified from σA-weighted 2Fo–Fc and Fo–Fc electron density maps. The single GMPP was fitted into the density and refinement performed. The final Rcryst is 21.0% and Rfree is 24.2% at 2.1-Å resolution (SI Table 3).
Modeling of Human PGRP-IβC–GMPP2 Complex.
Two saccharides (NAG2 and NAM2) were added one by one to the PGRP-IβC–GMPP crystal structure, maintaining the β(1–4) glycosidic dihedral angles of C2(NAG1)–C1(NAG1)–O1(NAG1)–C4(NAM1) = 173° and C1(NAG1)–O1(NAG1)–C4(NAM1)–C3(NAM1) = 126°, which correspond to the NMR structure of GMPP2 (17). Each saccharide addition was followed by 200 cycles of steepest descent minimization and 300 cycles of conjugate gradient minimization in AMBER 7 (41). GMPP2 was allowed to move freely, the side chains of residues immediately surrounding GMPP2 were permitted to move, and the rest of PGRP-IβC was fixed. After two saccharides were added, the pentapeptide stem was attached to NAM2 with dihedral angles of 61° and −91° for N2(NAM2)–C2(NAM2)–C3(NAM2)–O3(NAM2) and C4(NAM2)–C3(NAM2)–O3(NAM2)–C9(d-lactyl) (17), and the entire structure was subjected to energy minimization.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM065248 (to G.-J.B.), AI047990, AI065612 (both to R.A.M.), and GM61629 (to S.M.).
Abbreviations
- Dap-type
diaminopimelate type
- GMPP
glucosamyl muramyl pentapeptide
- ITC
isothermal titration calorimetry
- Lys-type
l-lysine type
- MPP
muramyl pentapeptide
- NAG
N-acetylglucosamine
- NAM
N-acetylmuramic acid
- PGN
peptidoglycan
- PGRP
PGN recognition protein
- PGRP-IαC
C-terminal PGN-binding domain of human PGRP-Iα
- PGRP-IβC
C-terminal PGN-binding domain of human PGRP-Iβ.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 2EAV (for PGRP-IβC) and 2EAX (for PGRP-IβC-GMPP)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0701453104/DC1.
References
- 1.Medzhitov R, Janeway CA., Jr Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 3.Schleifer KH, Kandler O. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle RJ, Dziarski R. In: Molecular Medical Microbiology. Sussman M, editor. London: Academic; 2001. pp. 137–153. [Google Scholar]
- 5.Royet J, Dziarski R. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 6.Guan R, Mariuzza RA. Trends Microbiol. 2007;15:127–134. doi: 10.1016/j.tim.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Dziarski R, Platt KA, Gelius E, Steiner H, Gupta D. Blood. 2003;102:689–697. doi: 10.1182/blood-2002-12-3853. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Fraser IP, Fukase K, Kusumoto S, Fujimoto Y, Stahl GL, Ezekowitz RA. Blood. 2005;106:2551–2558. doi: 10.1182/blood-2005-02-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tydell CC, Yuan J, Tran P, Selsted ME. J Immunol. 2006;176:1154–1162. doi: 10.4049/jimmunol.176.2.1154. [DOI] [PubMed] [Google Scholar]
- 10.Lu X, Wang M, Qi J, Wang H, Li X, Gupta D, Dziarski R. J Biol Chem. 2006;281:5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer RI. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 12.Brogden KA. Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 13.Williams DH, Bardsley B. Angew Chem Int Ed. 1999;38:1172–1193. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1172::AID-ANIE1172>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Kahne D, Leimkuhler C, Wei L, Walsh C. Chem Rev. 2005;105:425–448. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- 15.Guan R, Roychowdhury A, Ember B, Kumar S, Boons G-J, Mariuzza RA. Proc Natl Acad Sci USA. 2004;101:17168–17173. doi: 10.1073/pnas.0407856101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan R, Brown PH, Swaminathan CP, Roychowdhury A, Boons G-J, Mariuzza RA. Protein Sci. 2006;15:1199–1206. doi: 10.1110/ps.062077606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meroueh SO, Bencze KZ, Hesek D, Lee M, Fisher JF, Stemmler TL, Mobashery S. Proc Natl Acad Sci USA. 2006;103:4404–4409. doi: 10.1073/pnas.0510182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rekharsky M, Hesek D, Lee M, Meroueh S, Inoue Y, Mobashery S. J Am Chem Soc. 2006;128:7736–7737. doi: 10.1021/ja061828+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbull WB, Daranas AH. J Am Chem Soc. 2003;125:14859–14866. doi: 10.1021/ja036166s. [DOI] [PubMed] [Google Scholar]
- 20.Swaminathan CP, Brown PH, Roychowdhury A, Wang Q, Guan R, Silverman N, Goldman WE, Boons G-J, Mariuzza RA. Proc Natl Acad Sci USA. 2006;103:684–689. doi: 10.1073/pnas.0507656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan R, Malchiodi EL, Wang Q, Schuck P, Mariuzza RA. J Biol Chem. 2004;279:31873–31882. doi: 10.1074/jbc.M404920200. [DOI] [PubMed] [Google Scholar]
- 22.Guan R, Wang Q, Sundberg EJ, Mariuzza RA. J Mol Biol. 2005;347:683–691. doi: 10.1016/j.jmb.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Byun M, Oh BH. Nat Immunol. 2003;4:787–793. doi: 10.1038/ni952. [DOI] [PubMed] [Google Scholar]
- 24.Chang CI, Pili-Floury S, Herve M, Parquet C, Chelliah Y, Lemaitre B, Mengin-Lecreulx D, Deisenhofer J. PLoS Biol. 2004;2:1293–1302. doi: 10.1371/journal.pbio.0020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiser JB, Teyton L, Wilson IA. J Mol Biol. 2004;340:907–917. doi: 10.1016/j.jmb.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 26.Chang CI, Ihara K, Chelliah Y, Mengin-Lecreulx D, Wakasuki S, Deisenhofer J. Proc Natl Acad Sci USA. 2005;102:10279–10284. doi: 10.1073/pnas.0504547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J-H, Kim M-S, Kim H-E, Yano T, Oshima Y, Aggarwal K, Goldman WE, Silverman N, Kurata S, Oh B-H. J Biol Chem. 2006;281:8286–8295. doi: 10.1074/jbc.M513030200. [DOI] [PubMed] [Google Scholar]
- 28.Chang CI, Chelliah Y, Borek D, Mengin-Lecreulx D, Deisenhofer J. Science. 2006;311:1761–1764. doi: 10.1126/science.1123056. [DOI] [PubMed] [Google Scholar]
- 29.Matter H, Szilagyi L, Forgo P, Marinic Z, Klaic B. J Am Chem Soc. 1997;119:2212–2223. [Google Scholar]
- 30.Vollmer W, Holtje JV. J Bacteriol. 2004;186:5978–5987. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodell EW. J Bacteriol. 1985;163:305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harz H, Burgdorf K, Holjte JV. Anal Biochem. 1990;190:120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- 33.Hesek D, Savorov M, Morio KI, Lee M, Brown S, Vakulenko S, Mobashery S. J Org Chem. 2004;69:778–784. doi: 10.1021/jo035397e. [DOI] [PubMed] [Google Scholar]
- 34.Hesek D, Lee MJ, Morio KI, Mobashery S. J Org Chem. 2004;69:2137–2146. doi: 10.1021/jo035583k. [DOI] [PubMed] [Google Scholar]
- 35.Roychowdhury A, Boons G-J. Tetrahedron Lett. 2005;46:1675–1678. [Google Scholar]
- 36.Wiseman T, Williston S, Brandts JF, Lin LN. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. 6. [DOI] [PubMed] [Google Scholar]
- 37.Colloborative Computational Project, Number 4. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.Peitsch MC. Biochem Soc Trans. 1996;24:274–279. doi: 10.1042/bst0240274. [DOI] [PubMed] [Google Scholar]
- 39.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 40.McRee DE. J Struct Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- 41.Case DA, Pearlman DA, Caldwell JW, Cheatham TE, III, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, et al. San Francisco: Univ of California; 2002. AMBER 7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.