Abstract
Previous studies have shown that insulin-like growth factor-1 (IGF-1) has beneficial effects, both clinically and histopathologically, on experimental autoimmune encephalomyelitis (EAE), although results vary depending on species and treatment regimen. The present study investigated whether IGF-1, delivered at different time points during the acute and chronic phases of adoptively transferred EAE in SJL mice, had the ability to affect or enhance myelin regeneration. Central nervous system tissue sampled at different stages of treatment was subjected to detailed neuropathological, immunocytochemical and molecular analysis. The results revealed some transient clinical amelioration and low level remyelination after IGF-1 administration during the acute phase of EAE. However, central nervous system tissue from acute phase treated animals sampled at chronic time points and from animals given IGF-1 during the chronic phase revealed no enhancing effect on remyelination in comparison to vehicle-treated controls. Examination of oligodendrocyte progenitor populations also revealed no differences between IGF-1- and vehicle-treated groups. At the cytokine level, the immunomodulatory molecules TGF-β2 and TGF-β3 displayed significant decreases that may have contributed to the transient nature of the effect of IGF-1 on EAE. Together with evidence from previous studies, it appears doubtful that IGF-1 is a good candidate for treatment in multiple sclerosis, for which EAE serves as a major model.
Experimental autoimmune encephalomyelitis (EAE), an inflammatory demyelinating disease of the central nervous system (CNS) and the prime animal model for multiple sclerosis (MS), can be induced by active or passive sensitization to myelin antigens and has been particularly useful in the testing of therapeutic compounds relevant to MS. 1,2 While attempts to abrogate EAE have traditionally involved immunological strategies and myelin antigens, it has become apparent in recent years that a number of pathways remote from autoimmune approaches may also have therapeutic relevance. One approach currently being explored in this regard is treatment of EAE with growth factors with known regulatory effects on myelinating cells. Among these, the neuregulin glial growth factor 2 (GGF2) has been shown to have a stimulatory effect on oligodendrocytes in vitro. 3 This feature led to its being tested in EAE where it was found to have a beneficial effect on the clinical and pathological outcome of disease, an effect corroborated by the observation of enhanced myelin repair, myelin gene up-regulation and suggestions of a switch from a proinflammatory to an immunoregulatory cytokine profile. 4
Another growth factor, insulin-like growth factor-1 (IGF-1), has been shown like GGF2, to promote oligodendrocyte development and stimulate myelin gene expression in vitro. 5,6 Previous studies in vivo have shown that IGF-1 is capable of reducing clinical signs and lesion severity in the Lewis rat and SJL mouse models of acute and chronic relapsing EAE. 7,8 Another laboratory has shown somewhat different results in the (PL × SJL/J) F1 mouse model of EAE, 9 where a delay in disease onset was seen after treatment during the acute phase. However, once signs developed, the disease was more severe with higher doses of IGF-1, and treatment of chronic animals had no effect. Since CNS remyelination is a major ultimate goal of many therapeutic protocols in MS, the present study was designed to compare the long-term effects of IGF-1 delivered during both the acute and chronic phases of chronic relapsing EAE from the standpoint of myelin gene expression and repair. The findings have shown some transient clinical improvement and low-level remyelination during the acute phase, but no clinical improvement and no difference in the level of remyelination between controls and groups chronically treated with IGF-1, although some indication of an influence on immunoregulatory cytokines was seen.
Materials and Methods
Animals and Induction of EAE
Female SJL/J mice (Jackson Laboratory, Bar Harbor, ME), were maintained in an NIH/AAALAC-approved facility. Donor mice were immunized at 5 to 12 weeks of age with an emulsion of 0.4 mg/ml bovine myelin basic protein (MBP; Sigma, St. Louis, MO), dissolved in PBS and emulsified with incomplete Freund’s adjuvant containing 60 μg of M. tuberculosis H37Ra (Difco Labs., Detroit, MI). Ten days later, lymph nodes were removed from these mice, the tissue homogenized into a single cell suspension, and the resultant cells cultured in the presence of 50 μg/ml of MBP for 3 days. These cells were then harvested, washed, counted and injected at a dose of 3 × 10 7 cells/0.2 ml into the tail vein of 5- to 6-week old naïve syngeneic recipients. Onset of signs in recipients occurred 6 to 10 days post-transfer (dpt) of cells. To prevent possible adverse physiological effects (hypoglycemia) in the IGF-1-treated groups, the diets of all animals were supplemented by the addition of sugar cubes to the regular mouse chow. Animals were graded according to a standard clinical index: 1 = limp tail; 2 = hind limb weakness; 3 = one limb plegic; 4 = plegia of 2 limbs; 5 = moribund or dead. 4 A total of 99 mice sensitized for EAE were examined for this study (51 IGF-1-treated, 48 control).
Treatment with IGF-1
Recombinant human IGF-1 (rhIGF-1) was provided by Genentech (S. San Francisco, CA). The IGF-1 was suspended in a vehicle solution consisting of 10 mmol/L acetic acid, 0.1 mol/L NaCl, and 0.1% Tween, pH 5.4. To determine dose, vehicle and two doses of rhIGF-1 (100 μg and 200 μg) in vehicle were tested using osmotic pumps implanted under the skin over the animal’s back on day 3 post-transfer (pt) of cells and removed 14 days later. Twenty-four animals were tested, 8 in each group, under code. Animals given 100 μg of IGF-1 displayed greater amelioration of EAE than the 200 μg IGF-1 group. Based on the better clinical outcome, a dose of 100 μg of IGF-1 was selected. Animals were treated by subcutaneous (s.c.) injection of the selected dose of IGF-1 or vehicle, delivered twice daily for 14 days before onset of signs (3 to 17 dpt), and at different stages (beginning at 25 to 78 dpt) of the chronic phase of EAE. Control and IGF-1-treated groups were observed clinically and scored under code for effects on disease onset, severity, and relapse rate.
Neuropathology
For neuropathological analysis, representative animals were sampled at selected time points during and after treatment. Ether-anesthetized mice were perfused with 2.5% glutaraldehyde in phosphate buffer. CNS tissue was removed and thin slices taken from 10 levels of the neuraxis (optic nerve, cerebrum, cerebellum/brainstem, cervical, thoracic, lumbar and sacral cord, and spinal nerve roots. These were postfixed in 1% osmium tetroxide for 1 hour, dehydrated, and embedded in epoxy resin. One-micrometer epoxy sections were stained with toluidine blue and examined by light microscopy in a blinded fashion by two investigators. A score from 0 to 5 was determined for inflammation, demyelination, remyelination, and Wallerian degeneration, based on the degree of CNS involvement, according to established criteria. 3,10 Remyelination was scored as follows: 1 = scattered thinly remyelinated fibers along the lesion margin; 2 = a narrow rim or small groups of remyelinated axons along the lesion margin; 3 = larger groups of remyelinating fibers; 4 = extensive remyelination of lesions; 5 = total remyelination. For electron microscopy (EM), thin sections were contrasted with uranyl acetate and lead citrate and examined in a Siemens 101.
Immunocytochemistry
Animals were perfused with PBS, the CNS removed, and slices from cerebral hemispheres, cerebellum, cervical, thoracic and lumbar cord embedded in OCT in a dry-ice bath. Frozen sections were fixed in acetone for 10 minutes and stained using the avidin-biotin-peroxidase complex technique (Vector Laboratories, Burlingame, CA). Overnight incubations were performed at 4°C with the following primary antibodies: rabbit anti-human IGF-1R (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:100 dilution; mouse anti-human IGF-1R (PharMingen, San Diego, CA) at 1:20; rabbit anti-human PDGF-R7 (a kind gift of C-H. Heldin of the Ludwig Institute for Cancer Research, Uppsala, Sweden) at 1:200; rabbit anti-rat NG2 (a kind gift of W. B. Stallcup, La Jolla, CA) at 1:100; anti-CNPase (Sigma) at 1:20; and rabbit anti-mouse MBP exon-2 (a kind gift of Dr. Carol Readhead, University of Southern California). Slides were scored in a blinded fashion by two observers on a scale of 0 to 4 based on the density of labeled cells.
Ribonuclease Protection Assay
For RNA preparation, anesthetized mice were perfused transcardially with ice-cold PBS and the spinal cord removed and snap-frozen in liquid nitrogen. Total RNA was extracted with tri-reagent (Molecular Research Center, Cincinnati, OH), as per manufacturer’s instructions using a Power Gen 125 homogenizer (Fisher Scientific), and adjusted to 5 μg/μl RNase-free H2O. The expression of various cytokine mRNAs was determined using a multiprobe RPA template set (Riboquant; PharMingen). A total of 20 μg total RNA from spinal cord was hybridized overnight to the [32P]UTP-labeled cRNA transcripts of the mCK-3b probe set using the RPA II Kit (Ambion, Austin, TX). Non-hybridized RNA was digested and the protected fragments were precipitated, dissolved in loading buffer and loaded on a denaturing 5% acrylamide gel. Bands were detected by autoradiography and quantified by phosphoimaging on a Storm 860 scanner using the ImageQuaNT 3.01 software package (Molecular Dynamics, San Francisco, CA). Results were calculated as a ratio of the volume of the band of interest to the sum of the bands for the housekeeping genes, L32 and GAPDH. Statistical significance was determined by Student’s t-test and ANOVA with a threshold value of P < 0.01.
Statistical Methods
The effect of treatment on clinical outcome was assessed by Student’s t-test and when the data were not normally distributed, the Mann-Whitney sum of ranks test.
Results
Clinical Findings
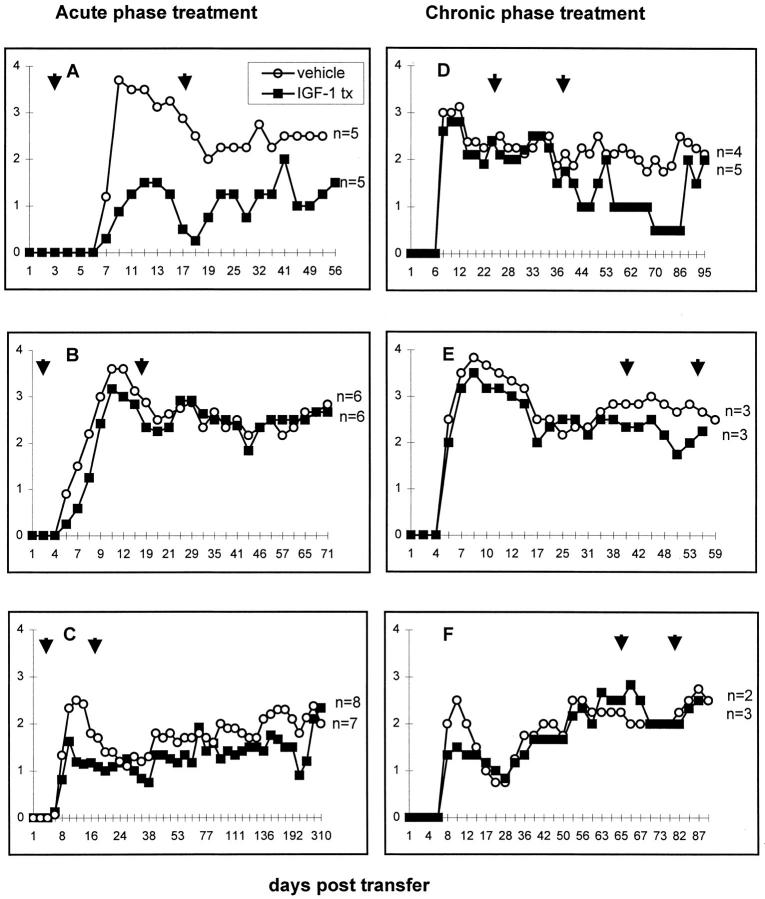
The clinical charts of six representative experimental groups are shown in Figure 1 ▶ . There were no significant differences in body weight between treated and control groups.
Figure 1.
Clinical charts of three experiments showing mice treated during the acute phase of EAE (A–C) and during the chronic phase (D–F). Arrows indicate period of treatment. In A and B, vehicle- and IGF-1-treated groups showed significantly different mean clinical scores at several time points. In A, using the Student t-test, IGF-1 provided protection at day 7 (P = 0.024), day 14 (P = 0.021), and day 17 (P = 0.041) and using the Mann-Whitney sum of rank analysis, on day 10 (P = 0.0l6). In B, the scores were significantly different on day 6 (P = 0.009, Mann-Whitney), and on day 7 (P = 0.022, t-test). Among the chronic phase groups, no significant differences were seen. y axis, clinical grade 1–4; x axis, days after transfer of cells.
IGF-1 Treatment during the Acute Phase
Mice treated with rhIGF-1 preceding and during the acute phase of adoptively transferred EAE (2 × 100 μg rhIGF-1 per diem, s.c. for 14 days beginning 3 dpt), displayed a less severe clinical course commencing on the same day of onset as vehicle-treated animals (4–6 dpt) (Figure 1) ▶ . In one experiment (Figure 1A) ▶ , the clinical course of the IGF-1-treated group with EAE (n = 5), remained at least one clinical grade lower than the vehicle-treated control group (n = 5), the differences being significant at day 7 (P = 0.024), day 14 (P = 0.021), day 17 (P = 0.041) using the t-test, and on day 10 (P = 0.016) using the Mann-Whitney sum of ranks test. However, in the subsequent experiment (Figure 1B) ▶ , the clinical scores for the two groups were different only at day 6 (P = 0.009, Mann-Whitney) and day 7 (P = 0.022, t-test). In the third experiment (Figure 1C) ▶ , there were no differences.
IGF-1 Treatment during the Chronic Phase
Three experiments were conducted during which mice with established chronic EAE were administered rhIGF-1 at different time points post-transfer (viz. days 25–39, days 41–55, and days 65–79 pt; Figure 1, D–F ▶ ). In the first experiment, the clinical course of the IGF-1-treated group (n = 5), appeared to improve posttreatment. In the second and third groups, the IGF-1-treated animals (n = 3), maintained courses similar to those of the vehicle-treated controls (Figure 1, D–F) ▶ .
Neuropathology
IGF-1 Treatment during the Acute Phase
At early time points during treatment, CNS lesions in IGF-1-treated animals were less inflammatory and demyelinative than in vehicle-treated controls, an observation correlating with the observed decreased clinical severity (Table 1 ▶ and Figure 1 ▶ ). However, with time, as the clinical course of both groups became similar, the histopathology was comparable to the extent that the two groups could not be distinguished. In general, however, control groups tended to show more Wallerian degeneration (axonal pathology), than treated groups during early time points (Table 1) ▶ . Lesions were typically distributed around the subpial regions of the lumbar spinal cord (Figure 2A) ▶ . Interestingly, while control animals sampled during the acute phase of disease had inflammatory demyelinating lesions, similar to IGF-1-treated groups, one animal from an IGF-1 group sampled on day 10 pt, displayed suggestions of low level remyelination (Figure 2, B and D) ▶ , with several fibers possessing disproportionately thin myelin sheaths around large diameter axons. These fibers existed in areas showing ongoing inflammation and demyelination. These same areas were examined by EM and remyelination was confirmed. The thin myelin sheaths were associated with numerous outer loops of oligodendroglial cytoplasm, (Figure 3,A and B) ▶ , features reminiscent of early myelination. 11 Control-treated and animals treated with IGF-1 during the acute phase and maintained long-term (up to 11 months pt), displayed spinal cord lesions that were indistinguishable from one another, being intensely gliotic with some residual inflammatory activity (Figure 2, E and F) ▶ . Such lesions contained numerous chronically demyelinated axons and a few remyelinated fibers toward the periphery. However, there was no difference in the degree of remyelination in both groups.
Table 1.
Pathology of EAE Mice Treated with IGF-1 during the Acute Phase
| Period of treatment | Day sampled | Treatment/control | Inflammation | Demyelination | Remyelination | Wallerian degeneration |
|---|---|---|---|---|---|---|
| 3–17 dpt | 10 | Tx | 2.4 ± 0.5 | 1.3 ± 1.1 | 0 | 0 |
| Con | 3.7 ± 0.7 | 2.7 ± 0.7 | 0 | 0 | ||
| 14 | Tx | 2.7 ± 0.7 | 1.5 ± 1.2 | 1.4 ± 0.5 | 1.0 ± 0 | |
| Con | 2.9 ± 0.6 | 2.5 ± 1.1 | 1.7 ± 0.5 | 2.2 ± 0.4 | ||
| 54 | Tx | 1.1 ± 1.0 | 0.8 ± 1.0 | 1.0 ± 0 | 0 | |
| Con | 2.7 ± 0.5 | 2 ± 0.8 | 2.0 ± 0 | 1.5 ± 0.5 | ||
| 3–17 dpt | 29 | Tx | 1.8 ± 0.6 | 1.0 ± 0.8 | 1.5 ± 0.5 | 1.8 ± 0.4 |
| Con | 2 ± 0.5 | 1.1 ± 0.8 | 1.8 ± 0.4 | 2.2 ± 0.9 | ||
| 71 | Tx | 2.1 ± 0.6 | 1.9 ± 1.0 | 1.2 ± 0.2 | 1.5 ± 0.5 | |
| Con | 1.8 ± 0.7 | 1.3 ± 0.7 | 0 | 0 | ||
| 3–17 dpt | 11 | Tx | 0 | 0 | 0 | 0 |
| Con | 2.8 ± 1.7 | 2.7 ± 2.6 | 1.0 ± 0 | 2.0 ± 0 | ||
| 11 mo | Tx | 1.8 ± 0.3 | 1.7 ± 0.9 | 1.0 ± 0 | 0 | |
| Con | 1.6 ± 0.5 | 1.6 ± 0.7 | 1.2 ± 0.4 | 0 |
Figure 2.
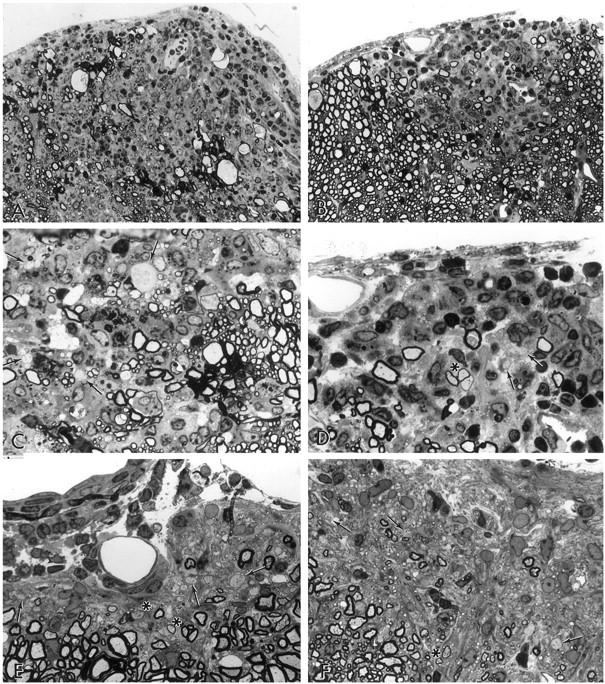
Pathology of lumbar spinal cord tissue from animals treated during the acute phase with IGF-1 and vehicle, sampled day 10 pt (A–D), and after 11 months pt (E–F). One-micrometer epoxy sections stained with toluidine blue. A: Vehicle-treated, 10 dpt, clinical score, grade 4. A large inflammatory demyelinated lesion is seen in an anterior column (×300). B: IGF-1-treated, 10 dpt, grade 1. Matching treated group for A. A less extensive inflammatory demyelinating lesion is seen in the anterior column. The asterisk indicates three fibers shown in detail in D (×300). C: Detail from A to show demyelinated axons (arrows) and macrophages containing myelin debris (×750). D: Same lesion as in B, higher magnification. The group of three fibers (shown in B) possess thin myelin sheaths suggestive of remyelination (asterisk). Demyelinated axons are also apparent (arrows) (×750). E: Vehicle-treated, 11 months pt, grade 3.5. A discrete zone of chronic demyelination and glial scarring in the subpial layer is seen. Note the glial scarring and demyelinated axons (arrows). A few remyelinated axons are present (asterisks) (×750). F: IGF-1-treated, 11 months pt, grade 2. In a matching animal, a lesion comparable in texture to E is seen. Demyelinated axons are shown at the arrows (×750).
Figure 3.
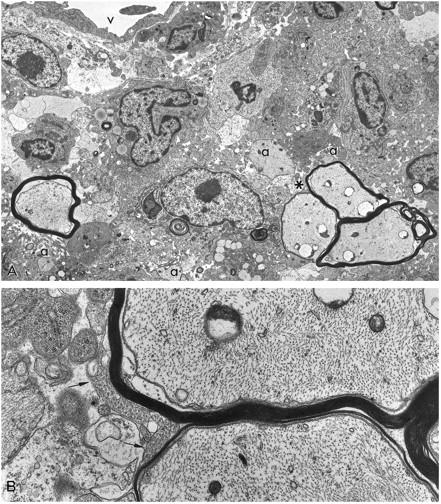
A: EM of a CNS lesion is shown from a mouse with EAE treated with IGF-1 during the acute phase and sampled day 10 pt (same field as shown in Figure 2, B and D ▶ ). The group of three thinly myelinated axons (asterisk) is seen to lie in the midst of a demyelinated, inflamed lesion. Vessel (V) above, demyelinated axons (a) (×3750). B: Detail of A illustrating the disproportionately thin myelin sheaths and oligodendroglial cytoplasmic tongues (arrows), features usually associated with ongoing myelination and remyelination (×16,000).
IGF-1 Treatment during the Chronic Phase
In all, five experiments were conducted in which mice that had developed chronic EAE were treated with IGF-1 and compared with vehicle-treated controls (Table 2) ▶ . Except for a reduced amount of inflammation in one group, IGF-1-injected animals treated from 25 to 39 dpt, the same group showing a clinical difference (Figure 1D) ▶ , there were no differences in any of the pathological parameters examined (inflammation, demyelination, remyelination and Wallerian degeneration) (Table 2) ▶ . Variation in pathology was noted in groups treated at different time points post-transfer, although there were no apparent effects attributable to IGF-1. For example, in one group given IGF-1 from 41 to 55 dpt and examined on day 60, both groups still displayed abundant inflammation and Wallerian degeneration while the levels of demyelination and remyelination were unremarkable (Figure 4, A and B) ▶ . In another group treated from 65–79 dpt and sampled on day 93, more typical gliotic and demyelinated lesions were seen (Figure 4, C–F) ▶ . None of the mice treated with IGF-1 during the chronic phase of EAE displayed a level of CNS remyelination significantly different from vehicle-treated controls.
Table 2.
Pathology of EAE Mice Treated with IGF-1 during the Chronic Phase
| Period of treatment | Day sampled | Treatment/control | Inflammation | Demyelination | Remyelination | Wallerian degeneration |
|---|---|---|---|---|---|---|
| 25–39 dpt | 38 | Tx | 1.7 ± 0.8 | 1.3 ± 1.0 | 0 | 0 |
| Con | 2.6 ± 0.7 | 1.2 ± 1.0 | 0 | 0 | ||
| 95 | Tx | 1.3 ± 0.5 | 1.1 ± 0.6 | 1.3 ± 0.5 | 0 | |
| Con | 2.2 ± 0.8 | 1.6 ± 0.2 | 1.8 ± 0.4 | 2.0 ± 0.9 | ||
| 30–44 dpt | 53 | Tx | 2.5 ± 1.8 | 1.1 ± 0.9 | 2.0 ± 1 | 2.3 ± 0.5 |
| Con | 2.0 ± 0.7 | 1.1 ± 0.8 | 1.4 ± 0.5 | 1.7 ± 0.4 | ||
| 41–55 dpt | 60 | Tx | 2.1 ± 0.7 | 1.9 ± 0.7 | 1.3 ± 0.5 | 2.6 ± 0.5 |
| Con | 2.2 ± 0.4 | 2.2 ± 1.2 | 1.3 ± 0.4 | 2.7 ± 0.7 | ||
| 65–79 dpt | 93 | Tx | 2.6 ± 0.7 | 2.3 ± 0.5 | 1.8 ± 0.4 | 2.5 ± 0.5 |
| Con | 2.2 ± 0.6 | 1.9 ± 0.9 | 1.3 ± 0.5 | 2.0 ± 0 | ||
| 78–92 dpt | 171 | Tx | 2.2 ± 1.5 | 1.3 ± 1.0 | 1.7 ± 0.5 | 0 |
| Con | 1.8 ± 0.8 | 1.7 ± 0.9 | 0 | 0 |
Figure 4.
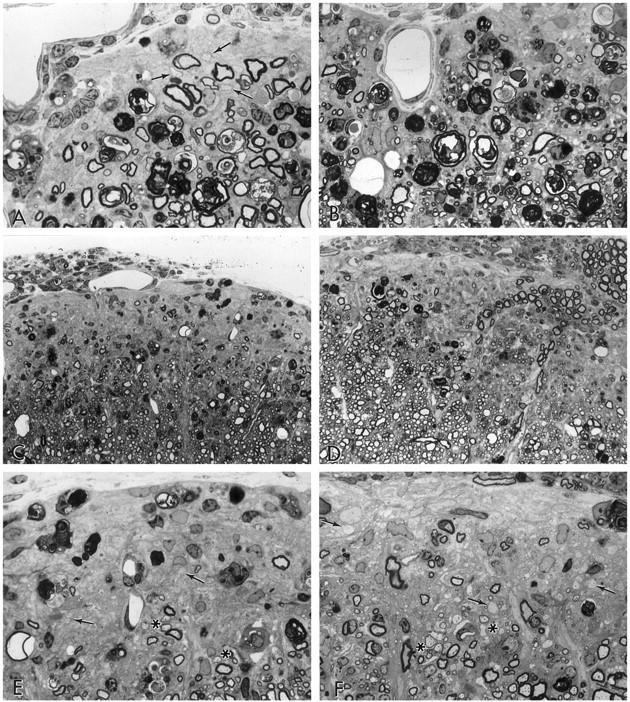
Lumbar spinal cord tissue from mice with EAE treated with vehicle and IGF-1 during the chronic phase (41–55 dpt), and sampled day 60 (A and B), and 65–79 dpt, sampled day 93 (C–F). A: Vehicle-treated, grade 3. A gliotic demyelinated lesion is seen in the subpial zone. Some Wallerian degeneration and a few remyelinated fibers (arrows) are present. Inflammatory cells are located within the meningeal space above (×750). B: IGF-1-treated, grade 2, matching animal to A. A small lesion lies along the subpial margin. Note the extensive glial scarring, Wallerian degeneration, and a few infiltrating cells above (×750). C: Vehicle-treated, grade 2.5. A large chronically demyelinated lesion lies in an anterior column. Some inflammation is seen in the leptomeninges above (×300). D: IGF-1-treated, grade 3.5; matching animal to C. A lesion similar to the control is shown. Note the inflammation in the leptomeninges, above, and the widespread gliosis (×300). E: Detail of C to show glial scarring, chronically demyelinated axons (arrows) and Wallerian degeneration. Remyelinated axons are seen at the asterisks (×750). F: Detail of D to show scattered chronically demyelinated axons (arrows) and a few remyelinated fibers (asterisks) (×750).
Immunocytochemistry for Early Oligodendrocyte Markers
As part of our study on the possible effects of IGF-1 on remyelination in this model, frozen sections immunoreacted for IGF-1R, PDGF-R, and MBP exon 2 transcripts were examined from all treated and control groups at different time points post transfer. In most cases, IGF-1R staining was seen on oligodendrocytes although the level of expression was low and similar between groups (Figure 5A) ▶ . Occasionally, astrocytes displayed immunoreactivity for IGF-1R. Staining for PDGF-R, a marker for progenitor oligodendrocytes, 12-14 was obtained in sections from both acute and chronic IGF-1-treated and control groups where scattered oligodendrocytes throughout the white matter displayed immunoreactivity (Figure 5B) ▶ . There was some increase in immunoreactivity for PDGF-R toward the margins of lesions but there were no differences in pattern at any time point between IGF-1 and control-treated mice. With a polyclonal rabbit antibody raised against mouse MBP exon 2 peptide, evidence for immature or remyelinating oligodendrocytes, 15 was sought in both IGF-1-treated and control mice, at early and late time points. In all animals, a few MBP exon 2-positive oligodendrocytes could be found at the margins of CNS lesions and scattered throughout the spinal cord white matter (Figure 5, C–F) ▶ . The numbers of MBP exon 2 positive cells were similar in IGF-1 and control-treated mice. Oligodendrocyte phenotype was confirmed with parallel sections stained for CNPase and MAG (Figure 4, G and H) ▶ .
Figure 5.
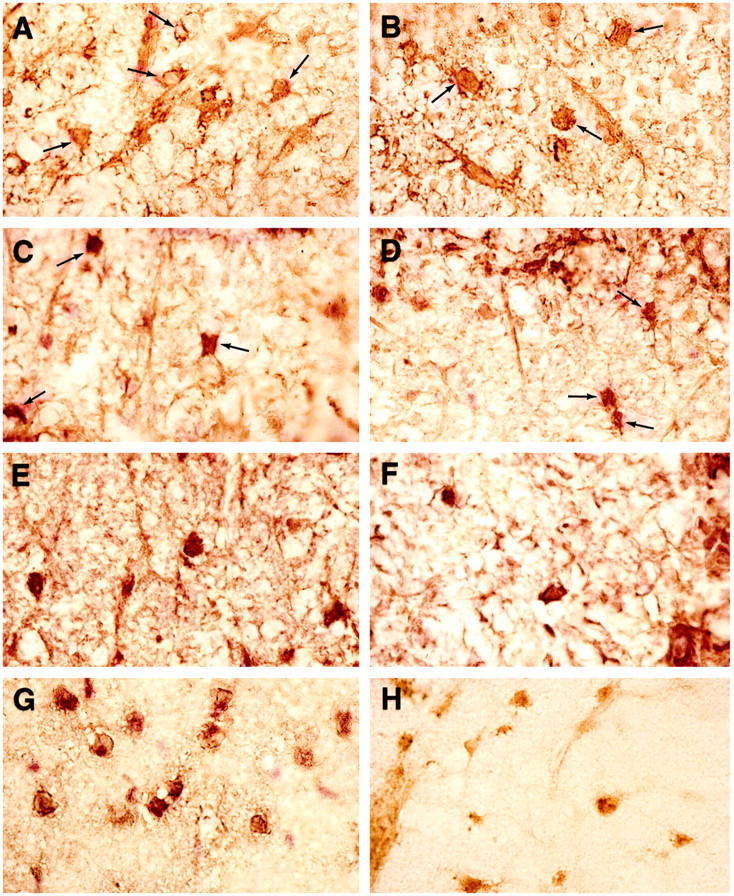
Lumbar spinal cord tissue from mice with EAE treated with IGF-1 or vehicle, immunoreacted for oligodendrocyte progenitors. No differences were observed between IGF-1 and control-treated groups at any time point. A: IGF-1 treatment during acute phase, sampled day 17 pt. Immunoreacted for IGF-1R. Several small rounded oligodendrocytes (arrows) show cytoplasmic staining (×750). B: Same animal as A, immunoreacted for PDGF-R7, a marker for oligodendrocyte progenitors. Several oligodendrocytes (arrows) display positive staining (×750). C: IGF-1 treatment, acute phase, sampled day 71 pt, immunoreacted for exon 2 MBP. Several oligodendrocytes (arrows) are intensely immunoreactive for this marker of immature oligodendrocytes (×750). D: Vehicle treatment, acute phase, sampled day 71 pt. Similar exon 2 MBP immunoreactivity is seen on two oligodendrocytes (×750). E: IGF-1 treatment, chronic phase (25–39 dpt), sampled day 52 pt, exon 2 MBP immunoreacted. A row of positively stained oligodendrocytes is seen (×750). F: Vehicle treatment, matching control for E, exon 2 MBP immunoreacted. Two oligodendrocytes stain intensely. Lesion activity is seen to the right (×750). G: Same animal as in A, CNPase immunoreacted. Note the intense staining of many oligodendrocytes (×750). H: Same animal as A, anti-MAG immunoreacted. Oligodendrocytes display moderate levels of immunoreactivity (×750).
RPA Assay
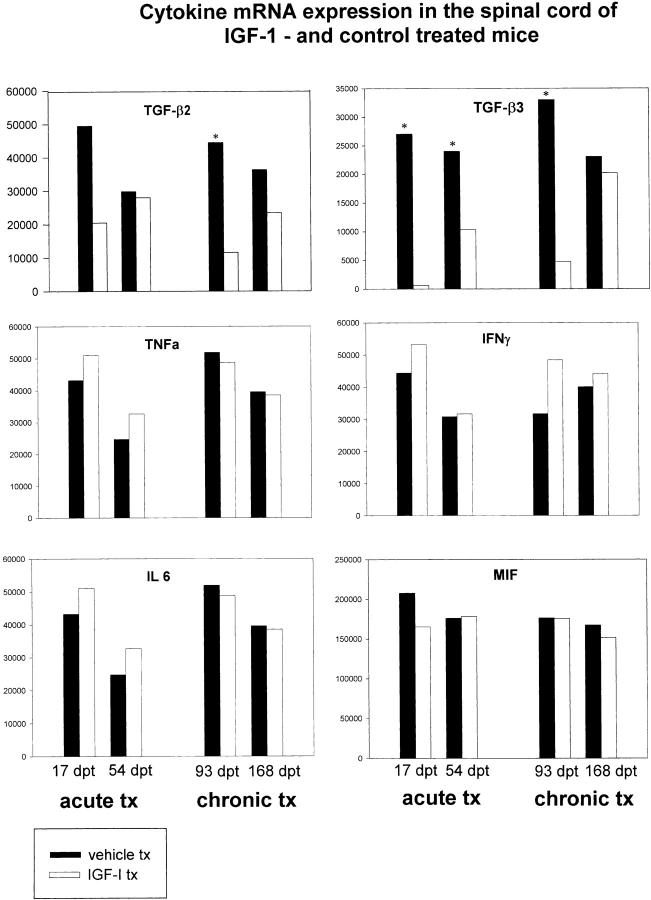
Data obtained with the mCK-3b set for cytokines from spinal cord mRNA from IGF-1-treated and control mice are shown (Figure 6) ▶ . No differences were noted for the expression of mRNA for TNF-α, IFN-γ, IL-6, and MIF. LT and TGF-β1 levels were not detectable in any of the samples. However, significant differences in the relative levels of mRNA were observed for TGF-β2 on day 93 pt of the chronic treatment group, and for TGF-β3 at both time points during acute treatment and the first time point in the chronic treatment groups (Figure 7) ▶ . The levels of these two cytokines were much reduced in IGF-1-treated mice.
Figure 6.
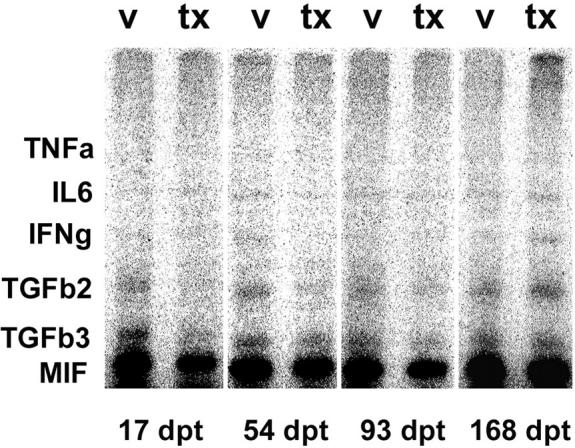
Multiprobe RPA analysis of cytokine mRNA expression in the spinal cord of IGF-1 (tx) and vehicle (v) treated mice measured at selected time points after treatment. The CNS was removed from saline-perfused animals and subjected to RPA analysis using the mCK-3b multiprobe template set (see Methods). Note that expression of TGF-β2 is decreased after treatment at one chronic time point. Also, TGF-β3 shows significant decreases after treatment. Figure 7 ▶ illustrates the quantitative analysis of these two cytokines, in addition to TNF-α, IFN-γ, IL-6, and MIF, none of which display significant changes.
Figure 7.
The gel shown in Figure 6 ▶ was phosphoimaged and the volumes of the protracted bands normalized to the bands for L32 and GAPDH. The data shown for TGF-β3 illustrates statistically significant differences in relative levels of mRNA between the vehicle- and IGF-1-treated animals at both time points, as is also the case for TGF-β2 at day 93 pt (asterisks).
Discussion
Based on the well known affinity of the growth factor, IGF-1, to promote proliferation and differentiation of oligodendrocytes in vitro, 5,6 and the promising previously demonstrated beneficial effects of this growth factor on the course of actively induced EAE, 7,16 the present study was initiated to test whether prolonged treatment at different stages of adoptively transferred EAE in SJL mice might influence myelin repair. The findings have shown that administration of IGF-1 to animals with EAE, during both the acute and chronic phases, had both positive and negative effects on disease outcome; positive, in that treatment before clinical onset clearly delayed and decreased both clinical signs and lesion formation, albeit transiently; and negative, in that clinical course and lesion histopathology eventually became indistinguishable between IGF-1 and control-treated animals. These findings are in agreement with a more recent study from another laboratory, 9 which showed that during treatment of acute EAE, IGF-1 was capable of reducing the clinical severity and the intensity of CNS inflammation, thus suggesting an effect on T cells, perhaps via IGF-1 receptors known to be expressed on these cells. 17,18 However, as stated above, in the present experiments, this increased efficacy was transient and the clinical and histopathological outcome in all groups ultimately became equivalent. It was demonstrated in a (PL×SJL/J) F1 mouse model of adoptive EAE 9 that a similar dose of IGF-1 had a mild protective effect when delivered in mice with acute EAE but, once disease developed, no significant effect. On the other hand, another laboratory showed that lower doses of IGF-1, resulted in decreases in relapse rate, lesion burden and inflammation after both acute and chronic treatment of SJL mice with adoptive EAE. However, enhanced remyelination, an anticipated finding based on earlier studies on EAE in the rat by the same group where IGF-1 treatment enhanced myelin protein synthesis, 16 was not found. The present study, which used more sensitive fine structural and immunocytochemical techniques for oligodendrocyte progenitors, was also unable to document any effect of IGF-1 on myelin regeneration.
That a previous 9 and the present study on the IGF-1 paradigm in EAE has demonstrated transient beneficial effects suggests a common mechanistic pathway. Previous workers 9 examined brain and spinal cord from experimental and control groups for adhesion molecule expression and found decreased ICAM-1 in IGF-1-treated mice. This, they suggested, may merely have reflected differences in inflammatory cell infiltration. On the other hand, it may have correlated with the delayed entry of cells into the CNS. Results from the same authors, using lymph node cells stimulated in vitro with MBP and/or IGF-1, revealed no significant differences in cytokine profile. From the present work, two cytokines displayed significant differences in expression in the CNS between experimental and control groups. These were TGF-β2 and TGF-β3 and the decreases in expression in the IGF-1-treated groups occurred at both acute and chronic time points. Whether this represents evidence for decreased immunomodulatory cytokine activity (at the mRNA level, at least), has relevance to the transient nature of the beneficial effect on disease severity, remains a possibility in need of further investigation. This is particularly apropos since TGF-β may play an important role in oligodendrocyte differentiation, 19 and is an effective therapeutic molecule in the treatment of acute and chronic relapsing EAE. 20-22 More recently, TGF-β2 has been reported to reduce demyelination in the Theiler’s virus model, 23 further testifying to its beneficial effect on CNS myelin. These observations may be relevant to the present findings in that the decrease in TGF-β2 and -β3 may be the indirect effect of IGF-1 acting on cells of the immune system and the CNS, where astrocytes have been demonstrated to synthesize TGF-β1, -β2, and -β3. 24
The observation of “precocious” remyelination seen after treatment during the acute phase of EAE may be genuine and related to a stimulatory effect on oligodendrocytes by IGF-1, as has been shown at the level of myelin gene expression in previous work on rat EAE. 16 However, it was encountered in a single animal only and may have been the result of a number of pleiotropic factors, as has been speculated in acute fulminant MS lesions where remyelination is also a feature, 25,26 the result perhaps of soluble mediators produced by macrophages. Nevertheless, the appearances were consistent with remyelination (as opposed to incomplete demyelination), since it was associated with elaboration of multiple oligodendrocyte processes. On the down side, this appeared to be a transient repair phenomenon, as is believed to be the case in MS. Gene expression by oligodendrocytes examined from the standpoint of progenitor cells and myelin demonstrated a lack of increased involvement of myelinating cells in IGF-1-treated mice.
Taken in concert, under the conditions studied, IGF-1 had a marked transient beneficial effect on the early course of EAE in the mouse when administered before clinical onset, but no measurable clinical or histopathological effect, particularly at the level of remyelination, when delivered during chronic disease. Since the latter situation is that which parallels most closely the course of human MS, it is therefore doubtful that IGF-1 would be efficacious in the human disease. Thus, while manipulation of immune-mediated demyelinating disease with growth factors with known propensities to cause oligodendrocyte stimulation or proliferation provides a novel approach which avoids complications arising during immune-mediated therapy, careful screening of each growth factor in different models remains essential.
Acknowledgments
We thank Dr. Ross Clark and Joffre Baker (Genentech, S. San Francisco, CA) for discussion; Everett Swanson, Miriam Pakingan, Howard Finch, and Debbie Mortensen, for expert technical assistance; and Ms. Patricia Cobban-Bond for preparation of the manuscript.
Footnotes
Address reprint requests to Dr. Barbara Cannella, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461-1602. E-mail: cannella@aecom.yu.edu.
Supported in part by National Multiple Sclerosis Society grant RG 1001-J-10; USPHS grants NS 08952, NS 11920 and NS 07098; a grant from Genentech Inc., S. San Francisco, CA; and the Wollowick Family Foundation.
References
- 1.Raine CS: Demyelinating diseases. Davis RL Robertson DM eds. Textbook of Neuropathology. 1990, :pp 535-620 Williams & Wilkins, Baltimore [Google Scholar]
- 2.Reder AT, Arnason BGW: Immunology of MS. Handbook of Clinical Neurology, 1985, vol. 3:pp 337-395 Elsevier, (47): Demyelinating Diseases. Edited by JC Koetsier. Amsterdam [Google Scholar]
- 3.Cannoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL: GGF/Neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron 1996, 17:229-243 [DOI] [PubMed] [Google Scholar]
- 4.Cannella B, Hoban CJ, Gao Y-L, Garcia-Arenas R, Lawson D, Marchionni M, Gwynne D, Raine CS: The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci USA 1998, 95:10100-10105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMorris FA, Dubois-Dalcq M: Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res 1988, 21:199-209 [DOI] [PubMed] [Google Scholar]
- 6.Shinar Y, McMorris FA: Developing oligodendroglia express mRNA for insulin-like growth factor-1, a regulator of oligodendrocyte development. J Neurosci Res 1995, 42:516-527 [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Yao D-L, Webster H: Insulin-like growth factor I treatment reduces clinical deficits and lesion severity in acute demyelinating experimental autoimmune encephalomyelitis. Multiple Sclerosis 1995, 1:2-9 [DOI] [PubMed] [Google Scholar]
- 8.Li W, Quigley L, Yao D-L, Hudson LD, Brenner M, Zhang BJ, Brocke S, McFarland HF, Webster H: Chronic relapsing experimental autoimmune encephalomyelitis: Effects of insulin-like growth factor-1 treatment on clinical deficits, lesion severity, glial responses, and blood-brain barrier defects. J Neuropathol Exp Neurol 1998, 57:426-438 [DOI] [PubMed] [Google Scholar]
- 9.Lovett-Racke AE, Bittner P, Cross AH, Carlino J, Racke MK: Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBPs). J Clin Invest 1998, 101:1797-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore GRW, Traugott U, Farooq M, Norton WT, Raine CS: Experimental autoimmune encephalomyelitis: Augmentation of demyelination by different myelin lipids. Lab Invest 1984, 51:416-424 [PubMed] [Google Scholar]
- 11.Raine CS: The morphology of myelin and myelination. Morell P eds. Myelin. 1984, :1-50 Plenum. New York [Google Scholar]
- 12.McMorris FA, McKinnon RD: Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol 1996, 6:313-329 [DOI] [PubMed] [Google Scholar]
- 13.Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P: Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 1988, 333:560-562 [DOI] [PubMed] [Google Scholar]
- 14.Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD: Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 1988, 333:562-565 [DOI] [PubMed] [Google Scholar]
- 15.Capello E, Voskuhl RR, McFarland HF, Raine CS: Multiple Sclerosis: implications of re-expression of a developmental gene (uc2 MBP) for repair of chronic lesions. Ann Neurol 1997, 41:797-805 [DOI] [PubMed] [Google Scholar]
- 16.Yao DL, Liu X, Hudson LD, Webster HdeF: Insulin-like growth factor-1 treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 1995, 92:6190–6194 [DOI] [PMC free article] [PubMed]
- 17.Clark R, Strasser J, McCabe S, Robbins K, Jardieu P: Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest 1993, 92:540-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tapson VF, Boni-Schnetzler M, Pilch DF, Center DM, Berman JS: Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor 1 in vitro. J Clin Invest 1988, 89:950-957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinnon RD, Piras G, Ida JA, Dubois-Dalcq M: A role for TGF-β in oligodendrocyte differentiation. J Cell Biol 1993, 121:1397-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE: Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-β1. J Immunol 1991, 146:3012-3017 [PubMed] [Google Scholar]
- 21.Johns LD, Flanders KC, Ranges GE, Sriram S: Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-β1. J Immunol 1991, 147:1792-1796 [PubMed] [Google Scholar]
- 22.Racke MK, Sriram S, Carlino J, Cannella B, Raine CS, McFarlin DE: Long-term treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor β2. J Neuroimmunol 1993, 46:175-184 [DOI] [PubMed] [Google Scholar]
- 23.Drescher KM, Murray PD, Lin X, Carlino J, Rodriguez M: TGF-β2 reduces demyelination, virus antigen expression and macrophage recruitment in a viral model of multiple sclerosis. J Immunol 2000, 164:3207-3213 [DOI] [PubMed] [Google Scholar]
- 24.Constam DB, Philipp J, Malipiero UV, Dijke P, Schachner M, Fontana A: Differential expression of transforming growth factor-β1, β2, and β3 by glioblastoma cells, astrocytes, and microglia. J Immunol 1992, 148:1404-1410 [PubMed] [Google Scholar]
- 25.Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho E-S: Multiple Sclerosis: Remyelination of nascent lesions. Ann Neurol 1993, 33:137-151 [DOI] [PubMed] [Google Scholar]
- 26.Raine CS, Wu E: Multiple Sclerosis: Remyelination in acute lesions. J Neuropathol Exp Neurol 1993, 52:199-205 [PubMed] [Google Scholar]