Summary
Recent studies have demonstrated a strong relationship between aging-associated reductions in mitochondrial function, dysregulated intracellular lipid metabolism, and insulin resistance. Given the important role of the AMP-activated protein kinase (AMPK) in the regulation of fat oxidation and mitochondrial biogenesis, we examined AMPK activity in young and old rats and found that acute stimulation of AMPK-α2 activity by 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) and exercise was blunted in skeletal muscle of old rats. Furthermore, mitochondrial biogenesis in response to chronic activation of AMPK with β-guanidinopropionic acid (β-GPA) feeding was also diminished in old rats. These results suggest that aging-associated reductions in AMPK activity may be an important contributing factor in the reduced mitochondrial function and dysregulated intracellular lipid metabolism associated with aging.
Keywords: HUMDISEASE
Introduction
The AMP-activated protein kinase (AMPK) is emerging as a chief regulator of whole-body energy balance (Bolster et al., 2002, Holmes et al., 1999, Kahn et al., 2005, Kimura et al., 2003, Krause et al., 2002, Terada et al., 2002, Winder et al., 1997, Winder et al., 2000). In skeletal muscle, once AMPK becomes activated, it exerts control in part by regulating fatty-acid oxidation through the phosphorylation of acetyl-CoA carboxylase 2 (ACC2) and mitochondrial biogenesis through increasing the expression of proteins vital for proper mitochondrial function such as citrate synthase and succinate dehydrogenase (Bergeron et al., 2001, Merrill et al., 1997, Winder et al., 1997, Winder et al., 2000, Zong et al., 2002). Reductions in mitochondrial oxidative-phosphorylation activity, which contribute to dysregulated intracellular lipid metabolism, have recently been implicated in the increased incidence of insulin resistance and type 2 diabetes that occurs with aging (Petersen et al., 2003). Given the critical role that AMPK plays in the regulation of mitochondrial biogenesis and fatty-acid oxidation, we examined the effect of aging on the AMPK signaling pathway and mitochondrial biogenesis during acute pharmacological stimulation, exercise, and chronic pharmacological stimulation.
Results and Discussion
AICAR Infusions
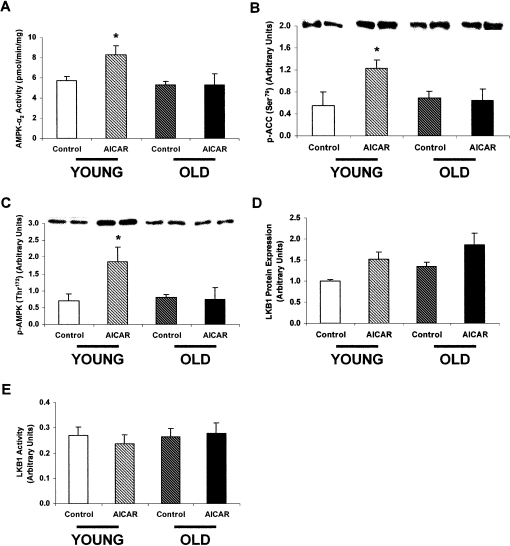
5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) is an acute activator of AMPK, which is taken up into cells and converted into 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside monophosphate (ZMP), which mimics the effects of AMP on AMPK (Corton et al., 1995). When bound to AMPK, AMP or ZMP induces a conformational change that makes AMPK a better substrate for activation by the upstream kinase LKB1 (Hawley et al., 2003). In vivo AICAR infusions in 3-month-old (“young”) and 28-month-old (“old”) Fisher 344 male rats increased AMPK-α2 activity by 44% in the extensor digitalis longus (EDL) muscles of the young AICAR-treated rats versus young saline-treated (control) rats (Figure 1A). In contrast, AICAR infusions did not increase AMPK-α2 activity in the old rats, demonstrating reduced AMPK activation with aging. Furthermore, AMPK phosphorylation of one of its key substrates in skeletal muscle, ACC2, at serine 79 (Ser79) was increased by 120% in the young AICAR-treated rats, whereas there was no difference in the old AICAR-treated rats (Figure 1B).
Figure 1.
The Effect of AICAR Infusions on AMPK and LKB1 in Young and Old Rats
(A) AMPK-α2 activity in the EDL muscle of young and old rats infused with either AICAR or saline (control). AMPK-α2 activity was increased by 44% in the young AICAR-treated rats compared to the young saline-treated rats. In contrast, there was no difference between the old AICAR-treated and saline-treated rats. (n = 7–10 in each group.) ∗p = 0.01. In this and all other figures, values are presented as means ± SEM.
(B) p-ACC (Ser79) in the EDL muscle of young and old rats infused with either AICAR or saline (control). AICAR infusion resulted in a 120% increase in p-ACC (Ser79) in the young rats. In contrast, there was no effect of AICAR infusion on p-ACC (Ser79) in the old rats. (n = 4–6 in each group.) ∗p < 0.05.
(C) p-AMPK (Thr172) in the EDL muscle of young and old rats infused with either AICAR or saline (control). AICAR infusion resulted in a 162% increase in p-AMPK (Thr172) in the young rats. In contrast, there was no effect of AICAR infusion on p-AMPK (Thr172) in the old rats. (n = 4–6 in each group.) ∗p < 0.05.
(D) LKB1 protein expression in the EDL muscle of young and old rats infused with either AICAR or saline (control). LKB1 protein expression in EDL muscle of young and old rats did not differ significantly during aging or in response to AICAR infusion. (n = 4 in each group.)
(E) LKB1 activity in the EDL muscle of young and old rats infused with either AICAR or saline (control). LKB1 activity in the EDL muscle of young and old rats did not differ significantly during aging or in response to AICAR infusion. (n = 4 in each group.)
AMPK activity is regulated by the phosphorylation of threonine 172 (Thr172) on the catalytic α subunit of the enzyme (Hawley et al., 1996), and studies in tissue-specific mouse knockouts suggest that LKB1 is the major upstream kinase in skeletal muscle, at least for α2 (Sakamoto et al., 2005). The reduced AICAR-stimulated AMPK-α2 activity could be attributed to reduced phosphorylation since Thr172 phosphorylation increased by 162% in the young AICAR-treated rats and was unaltered in the old AICAR-treated rats (Figure 1C). These aging-associated differences in AMPK-α2 activity and Thr172 phosphorylation between AICAR-treated and saline-treated rats were independent of any changes in AMPK-α1 activity or in the expression of the AMPK-α1 and α2 subunits (see Figure S1 in the Supplemental Data available with this article online). They also were not associated with changes in the expression (Figure 1D) or activity of LKB1 (Figure 1E) or in the phosphorylation of Ser491 on AMPK-α2 (data not shown), which has been reported to antagonize phosphorylation at Thr172 (Horman et al., 2006). LKB1 occurs as long and short polypeptides (Hawley et al., 2003), which have recently been identified as splice variants (S.A.H. and D.G.H., unpublished data). Although the two forms were expressed in almost equal amounts in rat skeletal muscle by western blotting, their expression was not different in the old rats.
Exercise
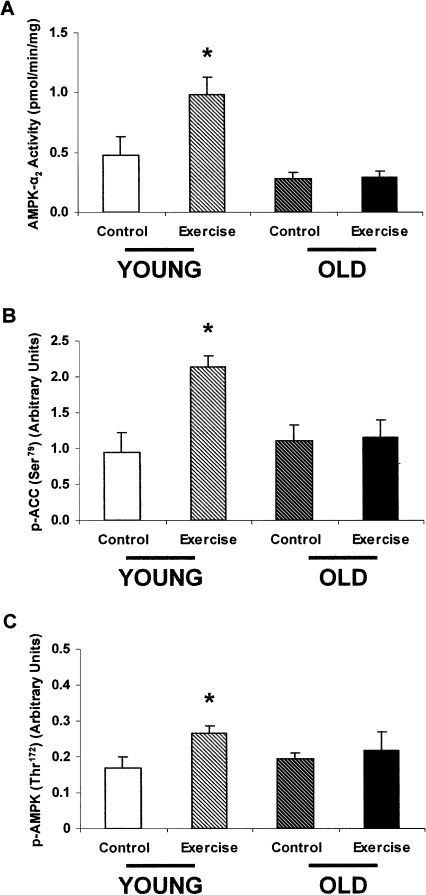
Exercise or contraction rapidly depletes ATP, which leads to the increase in the AMP:ATP ratio that is responsible for causing AMPK activation (Sakamoto et al., 2005, Winder and Hardie, 1996). Groups of young and old Fisher 344 male rats that had been exercise trained for 5 days completed a 5-day treadmill exercise regimen that resulted in a 110% increase in AMPK-α2 activity in the young rats. In contrast, the old rats displayed severely blunted AMPK-α2 activity in response to exercise (Figure 2A). Additionally, phosphorylation of Ser79 on ACC2 was blunted in the old rats despite a 127% increase in the young rats (Figure 2B). Lastly, Thr172 phosphorylation increased 55% in the young exercising rats and did not change in the old exercising rats compared to their controls (Figure 2C). These aging-associated differences in AMPK-α2 activity and Thr172 phosphorylation between exercising rats and sedentary rats were independent of any changes in AMPK-α1 activity or in the expression of the AMPK-α1 and α2 subunits (Figure S1).
Figure 2.
The Effect of Exercise on AMPK in Young and Old Rats
(A) AMPK-α2 activity in the EDL muscle of exercising or sedentary young and old rats. AMPK-α2 activity was increased by 110% in the young exercising rats compared to the young sedentary rats. In contrast, there was no difference between the old exercising rats and old sedentary rats. (n = 8 in each group.) ∗p = 0.01.
(B) p-ACC (Ser79) in the EDL muscle of exercising or sedentary young and old rats. Exercise resulted in a 127% increase in p-ACC (Ser79) in the young rats. In contrast, there was no effect of exercise on p-ACC (Ser79) in the old rats. (n = 4 in each group.) ∗p < 0.05.
(C) p-AMPK (Thr172) in the EDL muscle of exercising or sedentary young and old rats. Exercise resulted in a 55% increase in p-AMPK (Thr172) in the young rats. In contrast, there was no effect of exercise on p-AMPK (Thr172) in the old rats. (n = 4 in each group.) ∗p < 0.05.
β-GPA Feeding
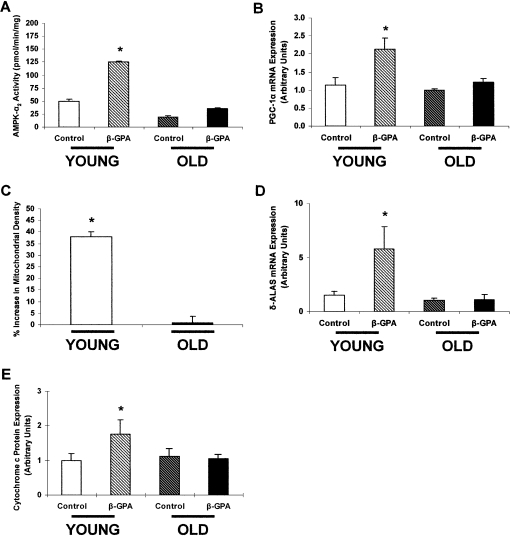
Recent studies have demonstrated that AMPK serves an important role in regulating mitochondrial biogenesis during chronic energy deprivation and is associated with increased mRNA expression of peroxisome proliferator-activated receptor γ coactivator 1α (Pgc-1α), the transcriptional coactivator responsible for mitochondrial biogenesis in skeletal muscle. To investigate how aging impacts AMPK-stimulated mitochondrial biogenesis, we chronically fed young and old rats a diet with 1% β-guanidinopropionic acid (β-GPA) for 8 weeks. Since β-GPA has a lower KM for creatine kinase than creatine, its presence results in lower intramyocellular concentrations of ATP and phosphocreatine and thus chronically stimulates AMPK activity. Accordingly, AMPK-α2 activity (Figure 3A) increased 146% in the EDL muscles of the young rats fed the β-GPA diet. A similar increase was detected in AMPK-α2 activity in the soleus tissue, an oxidative fiber, demonstrating the broad effect of β-GPA on AMPK-α2 activity in young rats. Pgc-1α mRNA expression (Figure 3B) also increased, by 86%, in the EDL muscles of the young rats fed the β-GPA diet. These increases in the young rats were associated with a 38% increase in mitochondrial density as assessed by electron microscopy (Figure 3C), a 289% increase in δ-ALA synthase (δ-Alas) mRNA expression (Figure 3D), and a 76% increase in cytochrome c protein expression (Figure 3E). In contrast, we observed no increases in AMPK-α2 activity, Pgc-1α mRNA expression, mitochondrial density, δ-Alas mRNA expression, or cytochrome c protein expression in the EDL muscles of the old rats. These results suggest that aging-associated reductions in AMPK-stimulated activity may be an important contributing factor in the reduced mitochondrial function and dysregulated intracellular lipid metabolism associated with aging-induced insulin resistance and type 2 diabetes.
Figure 3.
The Effect of β-GPA Feeding on AMPK and Mitochondrial Biogenesis in Young and Old Rats
(A) AMPK-α2 activity in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. The β-GPA-supplemented diet resulted in a 146% increase in AMPK-α2 activity in the young rats. In contrast, the β-GPA-supplemented diet had no effect on AMPK-α2 activity in the old rats. (n = 3–4 in each group.) ∗p < 0.01.
(B) Pgc-1α mRNA expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. Pgc-1α mRNA expression increased by 86% in the young β-GPA-fed rats. In contrast, there was no difference in Pgc-1α mRNA expression in the old rats. (n = 5–8 in each group.) ∗p < 0.05.
(C) Percent increase in mitochondrial density in the EDL muscle of young and old rats fed a β-GPA-supplemented diet compared to control rats. Mitochondrial density increased by 38% in the young β-GPA-fed rats. In contrast, the β-GPA-supplemented diet resulted in no difference in mitochondrial density in the old rats. (n = 4–5 in each group.) ∗p < 0.05.
(D) δ-Alas mRNA expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. δ-Alas mRNA expression increased by 289% in the young β-GPA-fed rats. In contrast, there was no difference in δ-Alas mRNA expression in the old rats. (n = 3–8 in each group.) ∗p = 0.05.
(E) Cytochrome c protein expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. Cytochrome c protein expression increased by 76% in the young β-GPA-fed rats. In contrast, there was no difference in cytochrome c protein expression in the old rats. (n = 4–8 in each group.) ∗p < 0.05 .
Experimental Procedures
In Vivo AICAR Infusions
We studied Fisher 344 male rats aged 3 months (young) and 28 months (old) (National Institute on Aging). The rats were maintained on standard rat chow (Ralston Purina) and housed in an environmentally controlled room with a 12/12 hr light/dark cycle. Body weight and food consumption were measured every 2–3 days. Rats were chronically catheterized via the right jugular vein and allowed to recover (5–8 days) until they regained their preoperative weight. Weight-matched young and old animals were infused with isotonic saline (control) or the AMPK activator 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) (bolus, 100 mg/kg; constant, 10 mg/kg/min; Toronto Research Chemicals Inc.) for 60 min. During the AICAR experiments, plasma glucose concentrations were maintained constant at basal concentrations (100 mg/dl) using a variable infusion of 20% (w/v) dextrose solution in order to prevent hypoglycemia. Blood was sampled at 0 min and every 15 min for glucose measurements. At the end of the infusions, rats were anesthetized with intravenous pentobarbital (50 mg/kg), and the skeletal muscle was rapidly excised and freeze clamped in liquid nitrogen.
Exercise
We studied Fisher 344 male rats aged 3 months (young) and 28 months (old). The rats were maintained on standard rat chow (Ralston Purina) and housed in an environmentally controlled room with a 12/12 hr light/dark cycle. Body weight and food consumption were measured every 2–3 days. The rats were introduced to the treadmill (Columbus Instruments) with a 10 min run at 10 m/min once per day for 4 days. A performance test to determine the maximum running capacity for each rat was performed on the fifth day by running the rats for 10 min at 10 m/min and then increasing the speed 1 m/min every minute until exhaustion. The young rats' maximum capacity was determined to be 38 m/min, and the old rats' maximum capacity was 20 m/min. The speed at which all of the rats reached exhaustion was noted, and the rats were run at 85% of that speed for 5 more days after a 2 day break for 30 min each day. Thus, the young rats were run at 32 m/min, and the old rats were run at 17 m/min. On the fifth day, immediately after exercise, the rats were anesthetized with isoflurane, and the skeletal muscle was rapidly excised and freeze clamped in liquid nitrogen. Throughout this experiment, control rats remained sedentary in their cages.
β-GPA Feeding
We studied Fisher 344 male rats aged 3 months (young) and 28 months (old). The rats were maintained on standard rat chow (Ralston Purina) with or without 1% β-GPA and were housed in an environmentally controlled room with a 12/12 hr light/dark cycle. Body weight and food consumption were measured every 2–3 days. On the eighth week of feeding, the rats were chronically catheterized via the right jugular vein and allowed to recover (5–8 days) until they regained their preoperative weight. The rats were then anesthetized with intravenous pentobarbital (50 mg/kg), and the skeletal muscle was rapidly excised and freeze clamped in liquid nitrogen for analytical procedures. Other skeletal muscle was prepared separately for mitochondrial quantification using electron microscopy.
Electron Microscopy
Individual skeletal muscle samples were prepared by immersion in 2.5% glutaraldehyde (Electron Microscopy Sciences) in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight. Skeletal muscles were then washed three times in 0.1 M cacodylate buffer (pH 7.4) and postfixed for 1 hr in 1% osmium tetroxide (EMbed-812 epoxy resin) in the same buffer at room temperature. After three washes in water, the samples were stained for 1 hr at room temperature in 2% uranyl acetate, washed again in water, and dehydrated in a graded series of ethanol dilutions (50%–100%). The samples were then embedded in EMbed. Ultrathin (60 nm) sections were cut using a Reichert Ultracut ultramicrotome, collected on formvar- and carbon-coated grids, stained with 2% uranyl acetate and lead citrate, and examined in a Philips 410 electron microscope. Only cross-sections of skeletal muscle were examined for quantification of mitochondrial density. For each individual rat and skeletal muscle, six random pictures were taken at a magnification of 7,100× and printed at a final magnification of 18,250×. The volume density of mitochondria was estimated using the point-counting method. The average volume density was calculated for each individual rat and skeletal muscle and was used to calculate the average volume density for each treatment.
AMPK Activity Assays
At the end of the experiments, the rats were anesthetized as described above. The EDL muscles from the left and right legs were quickly freeze clamped in situ. Skeletal muscle samples were kept in liquid nitrogen until analyzed. EDL was ground with a mortar and pestle and mixed with 1 ml of lysis buffer (50 mM Tris-HCl buffer [pH 7.5 at 4°C], 50 mM NaF, 5 mM NaPPi, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol [DTT], 1 mM benzamidine, 1 mM phenylmethanesulfonyl fluoride [PMSF], glycerol [10% v/v], Triton X-100 [1% v/v]). Homogenates were spun at 20,800 × g for 10 min at 4°C, and protein concentrations were determined. AMPK-α1 and AMPK-α2 were immunoprecipitated overnight from cell lysates containing 1 mg of protein using 1 μl of either AMPK-α1 antibody or AMPK-α2 antibody (Santa Cruz Biotechnology). Skeletal muscle AMPK-α1 and AMPK-α2 activity were determined by following the incorporation of [32P]ATP into a synthetic peptide containing the AMARA sequence on the following day.
LKB1 Activity Assays
EDL muscle samples were frozen in liquid nitrogen and later ground with a mortar and pestle. For each sample, the ground paste was extracted in 1 ml of lysis buffer as described above. Homogenates were spun at 12,900 rpm for 10 min at 4°C, and protein concentration was estimated. LKB1 was immunoprecipitated from lysates containing 3 mg of protein using the N-terminal antipeptide antibody (Boudeau et al., 2003). LKB1 assays (1 mg of lysate per assay) were conducted using purified GST-AMPK-α1 catalytic subunit as substrate in shaking incubators as described previously for immunoprecipitate kinase assays of AMPK (Hardie et al., 2000).
Real-Time Quantitative PCR Analysis
Total RNA was isolated from the EDL muscle using the RNeasy Kit (QIAGEN). cDNA was prepared from 2 μg of RNA using the StrataScript RT-PCR kit (Strategene) with random hexamer primers according to the manufacturer's instructions. The resulting cDNA was diluted, and a 4 ng aliquot was used in a 40 μl PCR reaction using SYBR green and selected primers. The primers for δ-Alas were 5′-TTTGTGGACGAGGTCCATGCAGTA-3′ (forward) and 5′-GCATTCAGCTGACGAATGTGGCTT-3′ (reverse). The primers for cytochrome c were 5′-TCTTGACTTCCTGACCTTGGGCTT-3′ (forward) and 5′-ACTCCCGCTTCTTGTAGCTTTCCA-3′ (reverse). PCR reactions were run in duplicate and quantitated with an ABI Prism 7700 Sequence Detection System (Applied Biosystems). Cycle threshold values were normalized to β-actin mRNA expression.
Western Blotting
For all western blots except for LKB1 expression, 30 μg of cell lysate was diluted in LDS Laemmli sample buffer before SDS-PAGE. SDS gel electrophoresis was performed using precast Bis-Tris 4%–12% gradient polyacrylamide gels in the MOPS buffer system (Invitrogen). After transfer to nitrocellulose membranes, membranes were incubated in blocking buffer (5% milk) for 1 hr and immunoblotted with anti-p-AMPK (Thr172) antibody (Cell Signaling), anti-p-ACC (Ser79) antibody (Cell Signaling), anti-AMPK-α1 antibody (Cell Signaling), anti-AMPK-α2 antibody (Cell Signaling), or cytochrome c antibody (Santa Cruz Biotechnology) at 1:1000 dilution in 5% bovine serum albumin. The membranes were washed three times for 15 min with TBS (10 mM Tris-HCl [pH 7.4], 0.5 M NaCl) plus Tween 20 (0.2% v/v) (TBST). The membranes were immersed in blocking buffer and a corresponding IgG-conjugated secondary antibody and were shaken for 1 hr. The membranes were then washed three times for 5 min using TBST. Proteins were then detected with enhanced chemiluminescence, and autoradiographs were quantified using densitometry. For western blotting of LKB1, 15 μg of tissue extract was diluted in LDS sample buffer (Invitrogen). SDS gel electrophoresis was performed using precast Bis-Tris 4%–12% gradient polyacrylamide gels in the MOPS buffer system (Invitrogen). Proteins were transferred to nitrocellulose membranes (Bio-Rad) using the Xcell II Blot Module (Invitrogen). Membranes were incubated in Odyssey blocking buffer (Li-Cor) for 1 hr. The membrane was subsequently incubated with 1:1000 anti-LKB1 N-terminal antibody (raised against residues 24–36), which had been diluted in Odyssey blocking buffer containing 0.2% Tween 20. The membranes were washed six times for 5 min with TBST. The membranes were immersed in blocking buffer containing Tween 20 (0.2% v/v) and 1 μg/ml anti-sheep IgG conjugated to IR dye 680 (Molecular Probes) and shaken for 1 hr, protected from light. The membranes were then washed six times for 5 min using TBST and once for 5 min in PBS. The membranes were scanned in the 700 nm channel using the Odyssey IR imager, and LKB1 protein levels were quantified using Odyssey IR imaging system software.
Statistical Analyses
All data are reported as means ± SEM. Results were analyzed using Student's t test. Differences were considered statistically significant at p < 0.05.
Acknowledgments
We thank Christina Horensavitz for her expertise with electron microscopy. This work was supported by grants P30 DK-45735, R01 DK-40936, U24 DK-59635, R01 HL-63811, and T32 GM-07499 from the US Public Health Service. G.I.S. is the recipient of a Distinguished Clinical Scientist Award from the American Diabetes Association. K.J.M., S.A.H., and D.G.H. are supported by a Programme Grant from the Wellcome Trust (UK) and the EXGENESIS integrated project (LSHM-CT-2004-005272) of the European Commission.
Published: February 6, 2007
Footnotes
Supplemental Data include one figure and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/5/2/151/DC1/.
Supplemental Data
References
- Bergeron R., Ren J.M., Cadman K.S., Moore I.K., Perret P., Pypaert M., Young L.H., Semenkovich C.F., Shulman G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Boudeau J., Baas A.F., Deak M., Morrice N.A., Kieloch A., Schutkowski M., Prescott A.R., Clevers H.C., Alessi D.R. MO25α/β interact with STRADα/β enhancing their ability to bind, activate, and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. 5-aminoimidazole-4-carboxamide ribonucleoside: A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;29:653–657. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Salt I.P., Davies S.P. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol. Biol. 2000;99:63–75. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- Hawley S.A., Davison M., Woods A., Davies S.P., Beri R.K., Carling D., Hardie D.G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley S.A., Boudeau J., Reid J.L., Mustard K.J., Udd L., Makela T.P., Alessi D.R., Hardie D.G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B.F., Kurth-Kraczek E.J., Winder W.W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., Schlattner U., Wallimann T., Carling D., Hue L., Rider M.H. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha subunits in heart via hierarchical phosphorylation of Ser485/491. J. Biol. Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. Published online December 9, 2005. [DOI] [PubMed] [Google Scholar]
- Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kimura N., Tokunaga C., Dalal S., Richardson C., Yoshino K., Hara K., Kemp B.E., Witters L.A., Mimura O., Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Krause U., Bertrand L., Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur. J. Biochem. 2002;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- Merrill G.F., Kurth E.J., Hardie D.G., Winder W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D.L., DePietro L., Cline G.W., Shulman G.I. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., McCarthy A., Smith D., Green K.A., Hardie D.G., Ashworth A., Alessi D.R. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S., Goto M., Kato M., Kawanaka K., Shimokawa T., Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem. Biophys. Res. Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- Winder W.W., Hardie D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. Endocrinol. Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder W.W., Wilson H.A., Hardie D.G., Rasmussen B.B., Hutber C.A., Call G.B., Clayton R.D., Conley L.M., Yoon S., Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and cAMP-dependent protein kinase. J. Appl. Physiol. 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- Winder W.W., Holmes B.F., Rubink D.S., Jensen E.B., Chen M., Holloszy J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J., Shulman G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.