Abstract
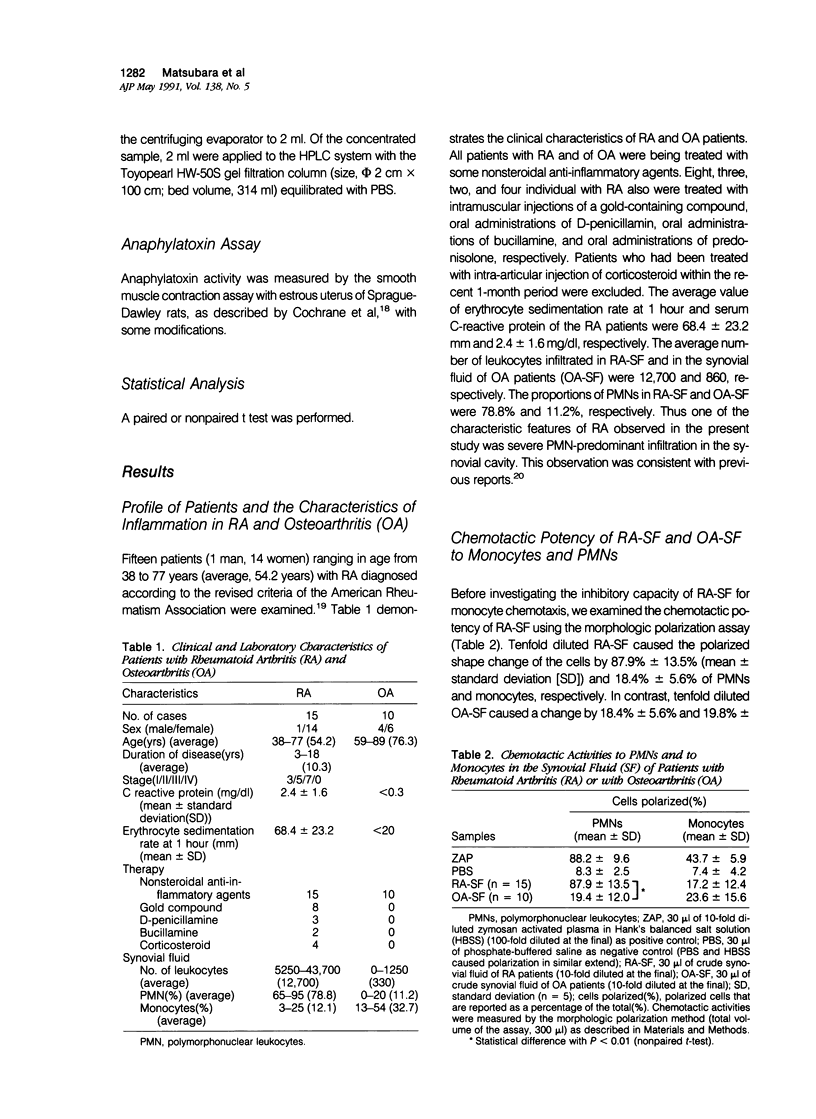
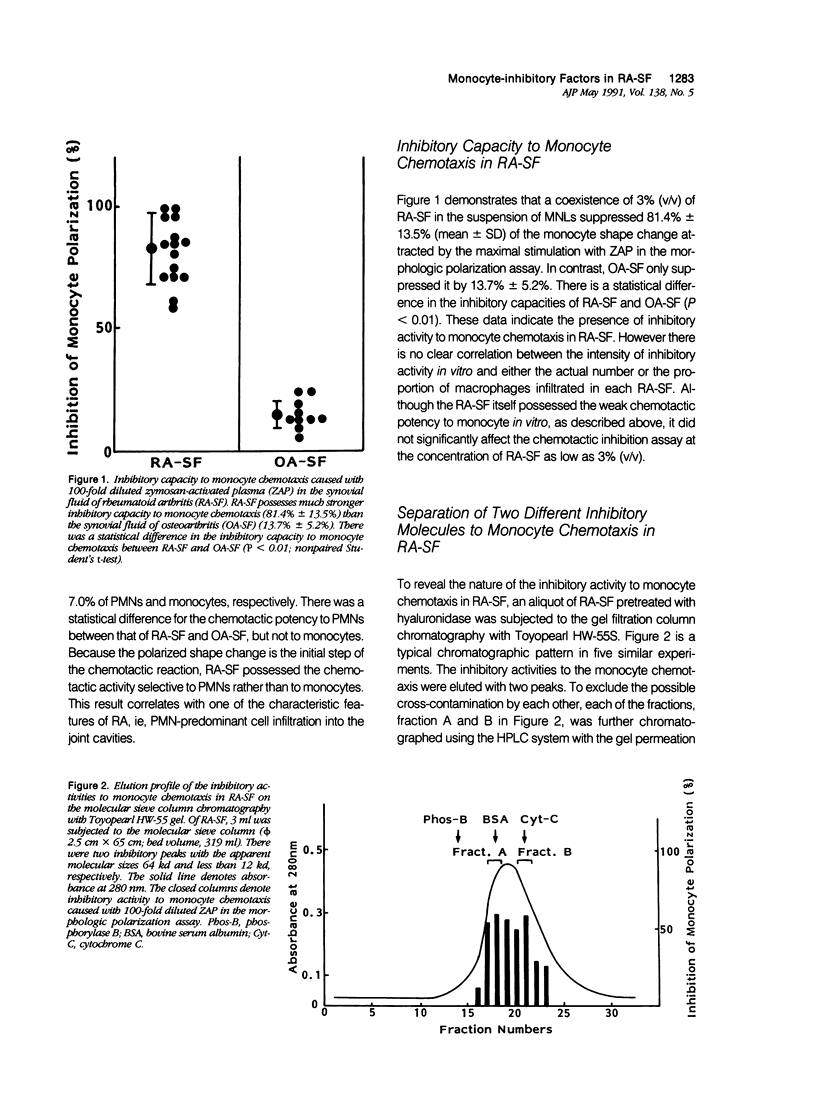
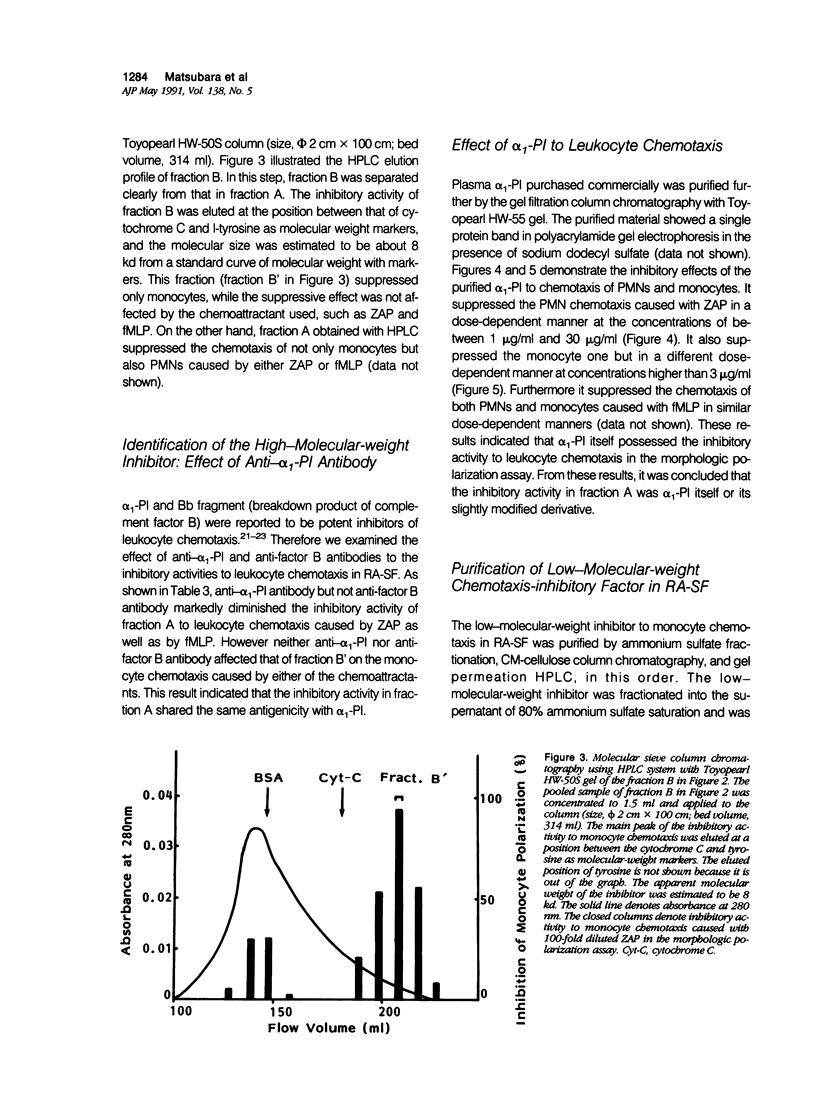
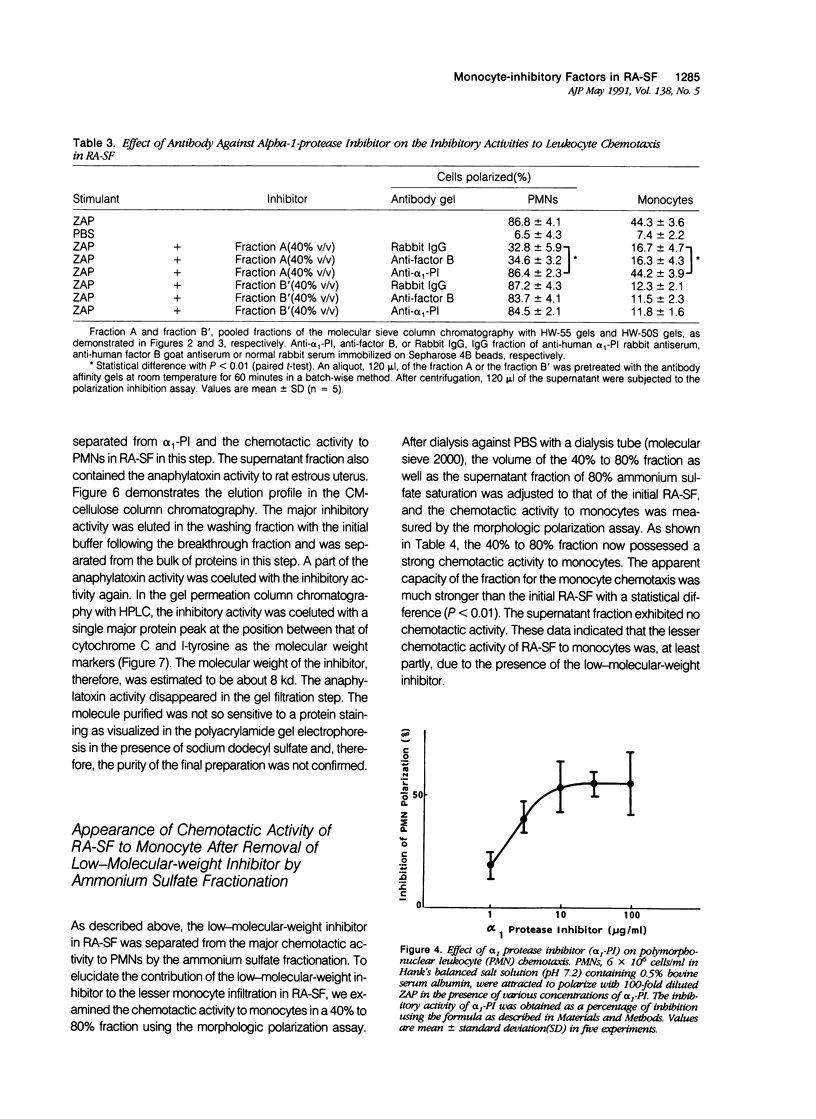
To reveal the mechanism of the lesser infiltration of monocytes in synovial cavities with rheumatoid arthritis despite the presence of chronic inflammation, the synovial fluid from 15 rheumatoid arthritis patients was analyzed with respect to leukocyte chemotaxis. The synovial fluid possessed strong chemotactic activity to polymorphonuclear leukocytes but rather suppressed one to monocytes. The synovial fluid contained two different inhibitory activities in monocyte chemotaxis. One, which also suppressed polymorphonuclear leukocyte chemotaxis, was identified as alpha 1 protease inhibitor. The other, with molecular weight of 8 kd, possessed the specificity to monocytes and shared the antigenicity with complement C4 but not with C3 or C5. A similar inhibitor was generated in normal human plasma when the classical pathway of the complement system was initiated with aggregated human IgG, while it was not when alternative pathway was initiated with zymosan. The small size factor in the synovial fluid, apparently derived from C4, seemed to be a cyto-directed factor that might block an early part of signal transduction system of monocytes in the chemotaxis. After removal of the small-size inhibitor, the synovial fluid exhibited chemotactic ability to monocytes. Therefore the apparent C4-derived factor might play a key role in the polymorphonuclear leukocyte-predominant infiltration in the synovial fluid of rheumatoid arthritis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Bianco C., Götze O., Cohn Z. A. Regulation of macrophage migration by products of the complement system. Proc Natl Acad Sci U S A. 1979 Feb;76(2):888–891. doi: 10.1073/pnas.76.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth W., Dunky A., Kleesiek K. Alpha 2-macroglobulin-proteinase complexes as correlated with alpha 1-proteinase inhibitor-elastase complexes in synovial fluids of rheumatoid arthritis patients. Arthritis Rheum. 1986 Mar;29(3):319–325. doi: 10.1002/art.1780290303. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. M., Rae S. A., Smith M. J. Leukotriene B4, a mediator of inflammation present in synovial fluid in rheumatoid arthritis. Ann Rheum Dis. 1983 Dec;42(6):677–679. doi: 10.1136/ard.42.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Goetzl E. J. Modulation of human neutrophil polymorphonuclear leucocyte migration by human plasma alpha-globulin inhibitors and synthetic esterase inhibitors. Immunology. 1975 Jul;29(1):163–174. [PMC free article] [PubMed] [Google Scholar]
- Gorski J. P., Hugli T. E., Müller-Eberhard H. J. C4a: the third anaphylatoxin of the human complement system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5299–5302. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad K. Presence of aggregated gamma-G-globulin in certain rheumatoid synovial effusions. Clin Exp Immunol. 1967 Jul;2(4):511–529. [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Gerard C., Kawahara M., Scheetz M. E., 2nd, Barton R., Briggs S., Koppel G., Russell S. Isolation of three separate anaphylatoxins from complement-activated human serum. Mol Cell Biochem. 1981 Dec 4;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- Kambara T., Kutsuna T., Okamoto T., Nakamura T. Inflammatory activities of synovial fluids from rheumatoid- and osteo-arthritis to guinea pig skin. Acta Pathol Jpn. 1982 Jul;32(4):575–583. doi: 10.1111/j.1440-1827.1982.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Klickstein L. B., Shapleigh C., Goetzl E. J. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J Clin Invest. 1980 Nov;66(5):1166–1170. doi: 10.1172/JCI109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid R. W., Grant R. F., Suquet C. M. Inhibition of equine neutrophil chemotaxis and chemokinesis by a Taenia taeniaeformis proteinase inhibitor, taeniaestatin. Parasite Immunol. 1987 Mar;9(2):195–204. doi: 10.1111/j.1365-3024.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Marder S. R., Chenoweth D. E., Goldstein I. M., Perez H. D. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985 May;134(5):3325–3331. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Okamoto T., Ueda K., Kambara T., Kutsuna T. Chemotactic activity for polymorphonuclear and mononuclear leukocytes in rheumatoid synovial fluids. Acta Pathol Jpn. 1986 Aug;36(8):1109–1122. doi: 10.1111/j.1440-1827.1986.tb02832.x. [DOI] [PubMed] [Google Scholar]
- PEKIN T. J., Jr, ZVAIFLER N. J. HEMOLYTIC COMPLEMENT IN SYNOVIAL FLUID. J Clin Invest. 1964 Jul;43:1372–1382. doi: 10.1172/JCI105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. The complement system in rheumatoid synovitis. I. An analysis of complement component activities in rheumatoid synovial fluids. Arthritis Rheum. 1970 Nov-Dec;13(6):713–723. doi: 10.1002/art.1780130601. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Fearon D. T., Austen K. F. Depressed synovial fluid levels of properdin and properdin factor B in patients with rheumatoid arthritis. Arthritis Rheum. 1975 Jul-Aug;18(4):289–295. doi: 10.1002/art.1780180401. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Fudman E. J. Demonstration of a chemotactic factor receptor on macrophages. J Immunol. 1980 Jun;124(6):2754–2757. [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Hausman M. H. A chemotactic factor for mononuclear leukocytes. Proc Soc Exp Biol Med. 1971 Nov;138(2):387–390. doi: 10.3181/00379727-138-35903. [DOI] [PubMed] [Google Scholar]
- Spilberg I., Mehta J., Daughaday C., Simchowitz L. Determination of a specific receptor for formyl-methionyl-leucyl-phenylalanine on th pulmonary alveolar macrophage and its relationship to chemotaxis and superoxide production. J Lab Clin Med. 1981 May;97(5):602–609. [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]


