Abstract
Superinfection of latently human immunodeficiency virus (HIV)-infected rabbits with either Treponema pallidum or Shope fibroma virus (SFV) activates HIV expression. In addition, HIV-infected rabbits demonstrate prolonged cutaneous lesions (chancres) after intracutaneous challenge with T. pallidum, the causative agent of syphilis. Rabbits were infected by intravenous inoculation of 3 x 10(7) human T-cell lymphotrophic virus type III (HTLV-III)/B10 (HIV-1)-infected H9 (human) cells. Five weeks after initial infection, integrated HIV-1-specific DNA sequences were detected in the DNA of the peripheral blood lymphocytes of only one of eight rabbits using polymerase chain reactions (PCR); human DNA could not be detected at this time. Furthermore HIV infection could not be demonstrated by either seroconversion or PCR during the next 6 months. All HIV-infected rabbits remained clinically healthy and had normal white blood cell counts. Six months after HIV infection, four HIV-infected and two noninfected controls were superinfected with 10(6) T. pallidum in eight skin sites in the shaved skin of the back, and four infected and two control animals were challenged with an intradermal injection with SFV. After infection with either syphilis or SFV, the DNA from the white blood cells of all eight HIV-infected rabbits contained HIV sequences, and HIV sequences were demonstrated in dermal mononuclear cells of the syphilitic lesions by in situ hybridization. The SFV-induced tumors were rejected normally in the HIV-infected rabbits, but four of the four rabbits challenged with T. pallidum had delayed development of cutaneous lesions and three of four demonstrated larger and more prolonged lesions. White blood counts, mitogen responses, and interleukin-2 production remained within normal limits, and seroconversion for HIV was not detected. Three of four rabbits in a second group, challenged with T. pallidum 4 months after HIV-inoculation, also had delayed healing of syphilitic lesions. These results indicate that latent HIV-infection of rabbits may be activated by immunostimulation and that latently HIV-infected rabbits have impaired delayed hypersensitivity reactions. It is hypothesized that true latent HIV-infection in the rabbits is in monocytes and postulated that further immunostimulation may produce infection of lymphocytes and activation of disease.
Full text
PDF



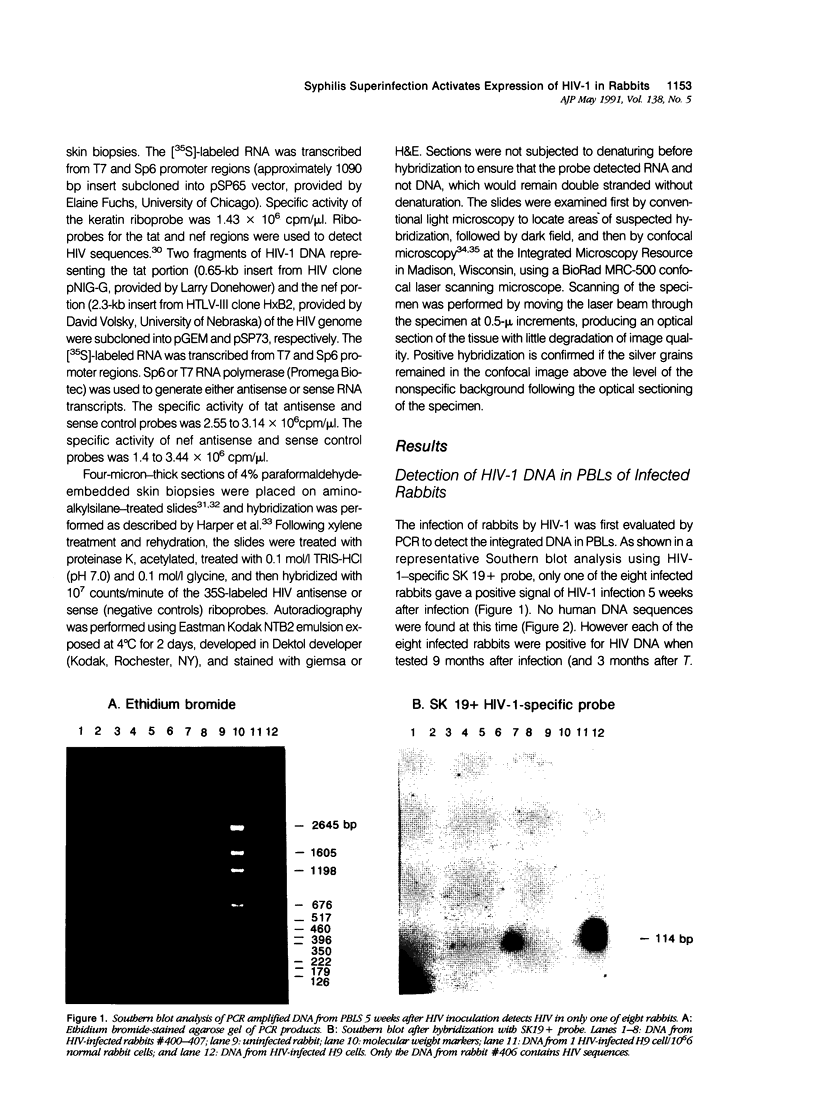
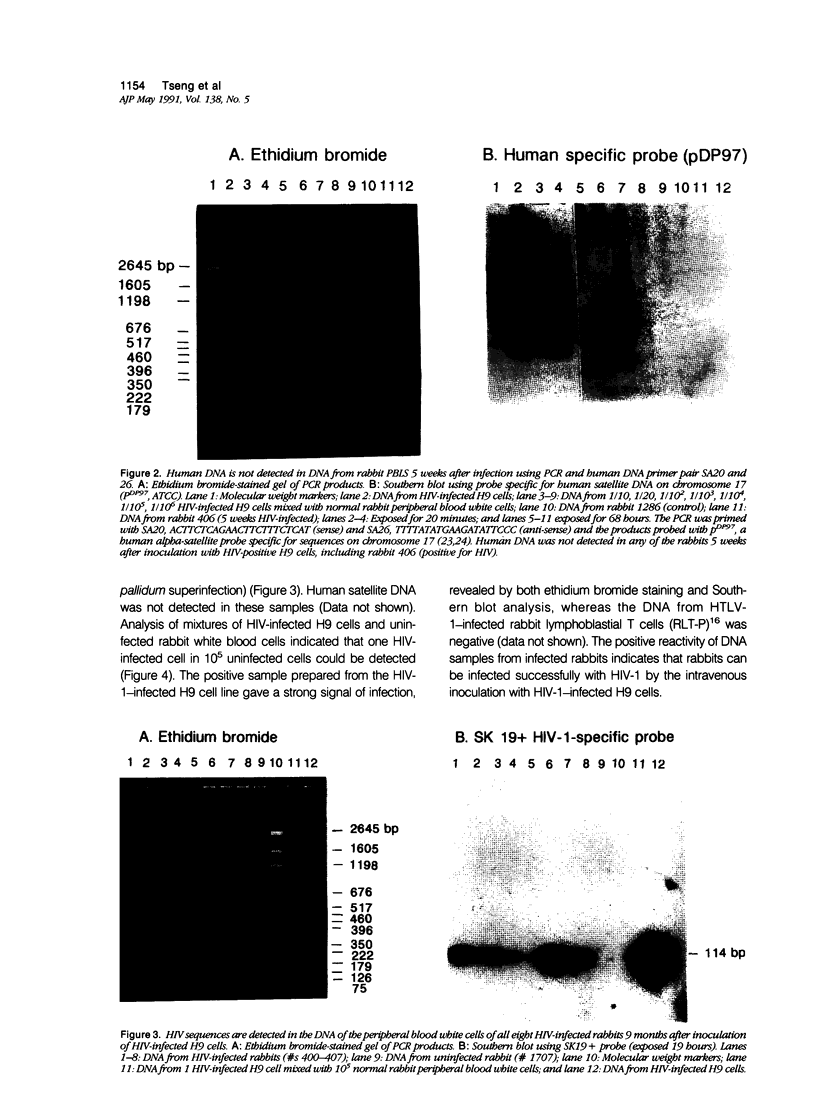
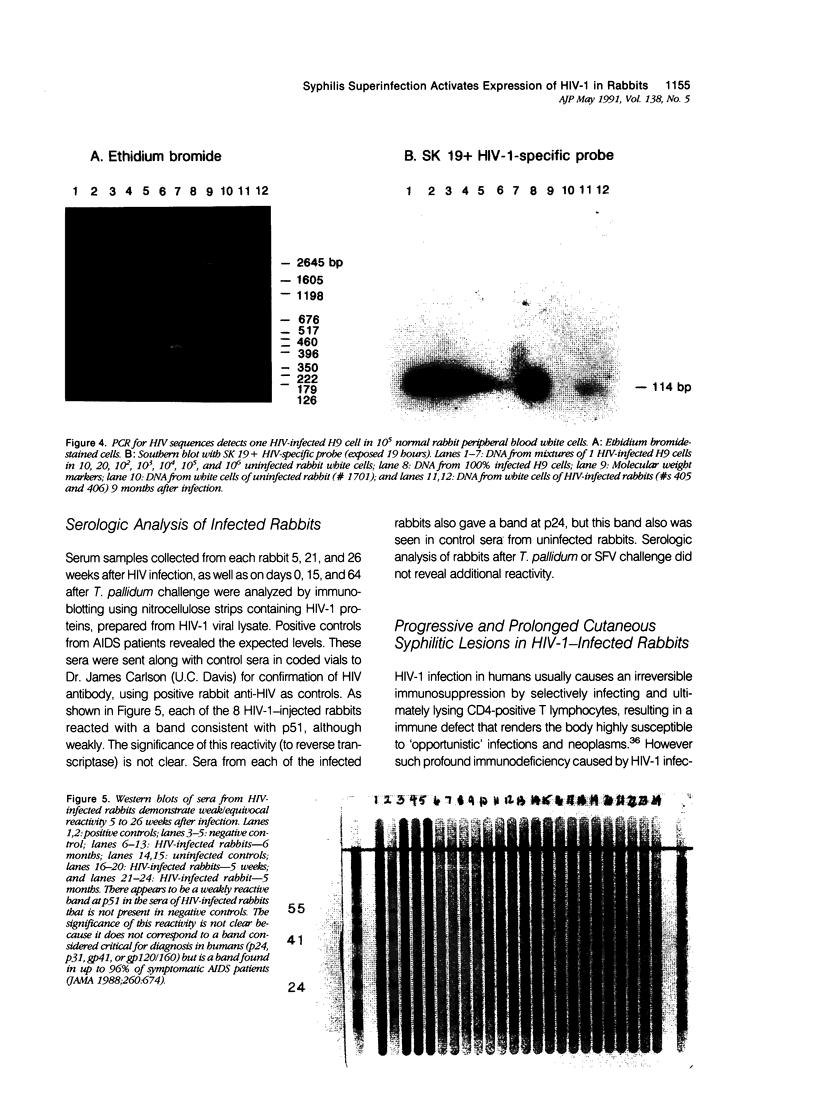
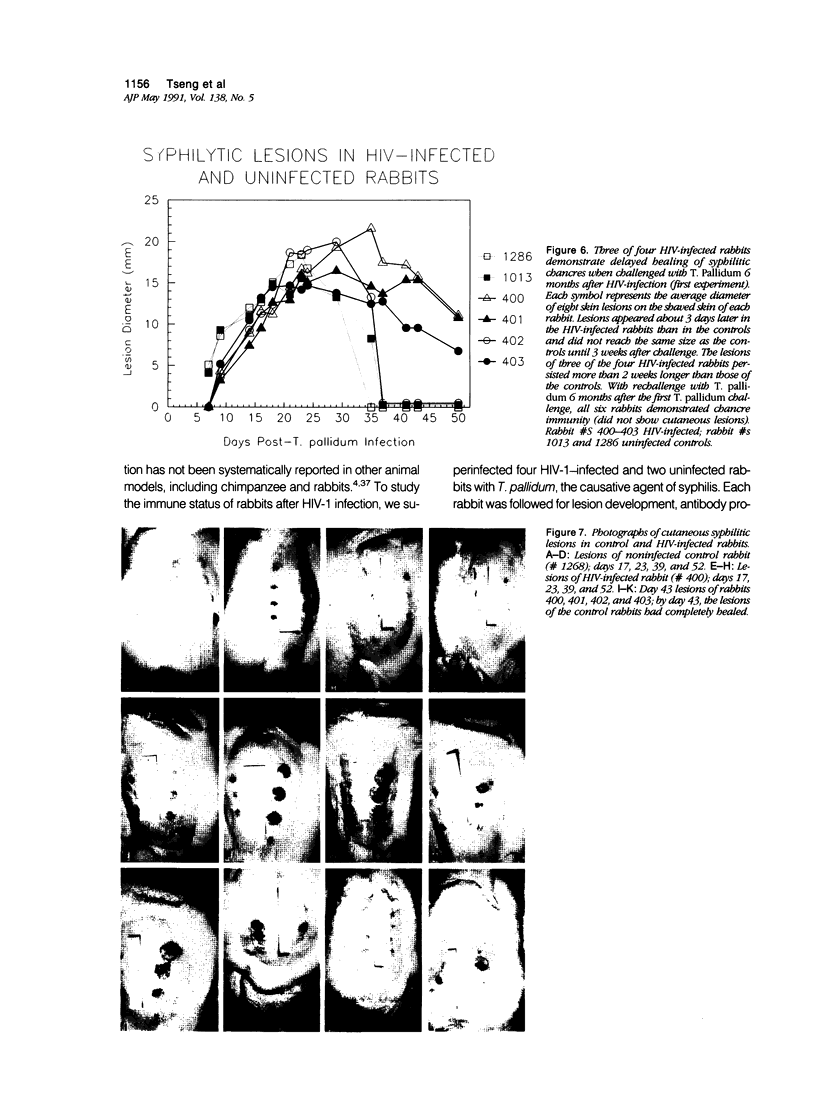
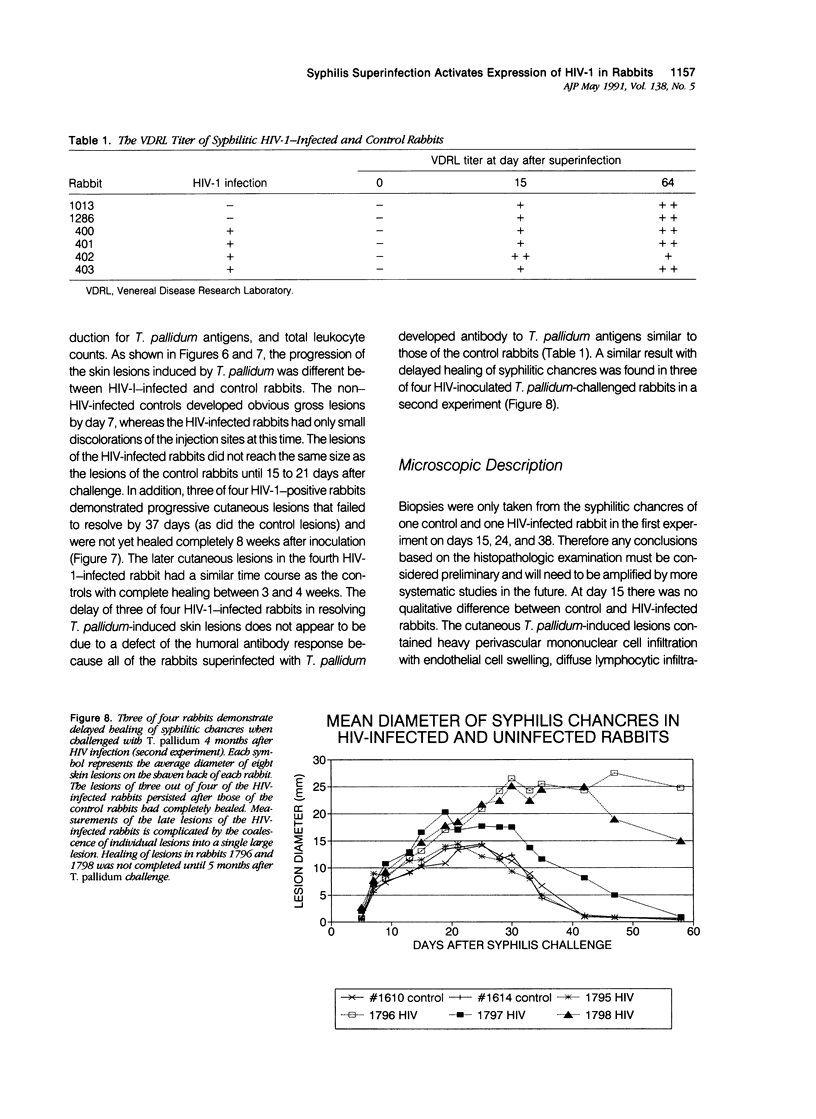

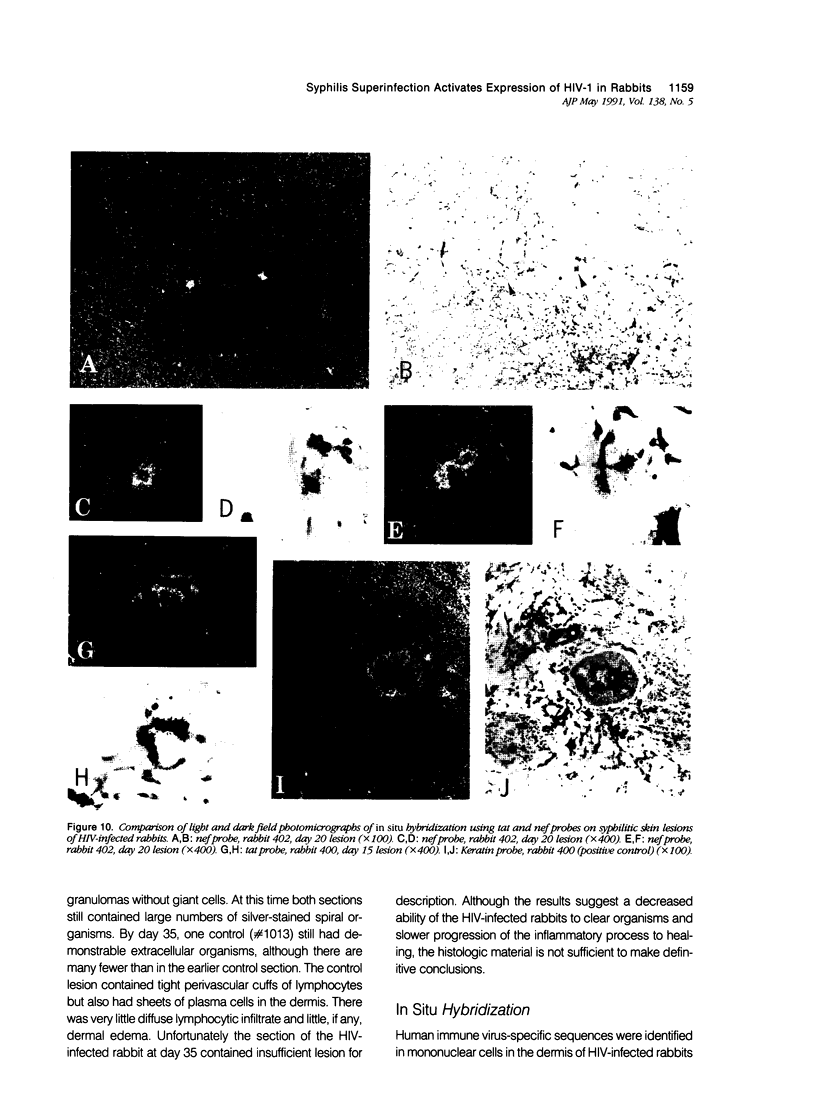

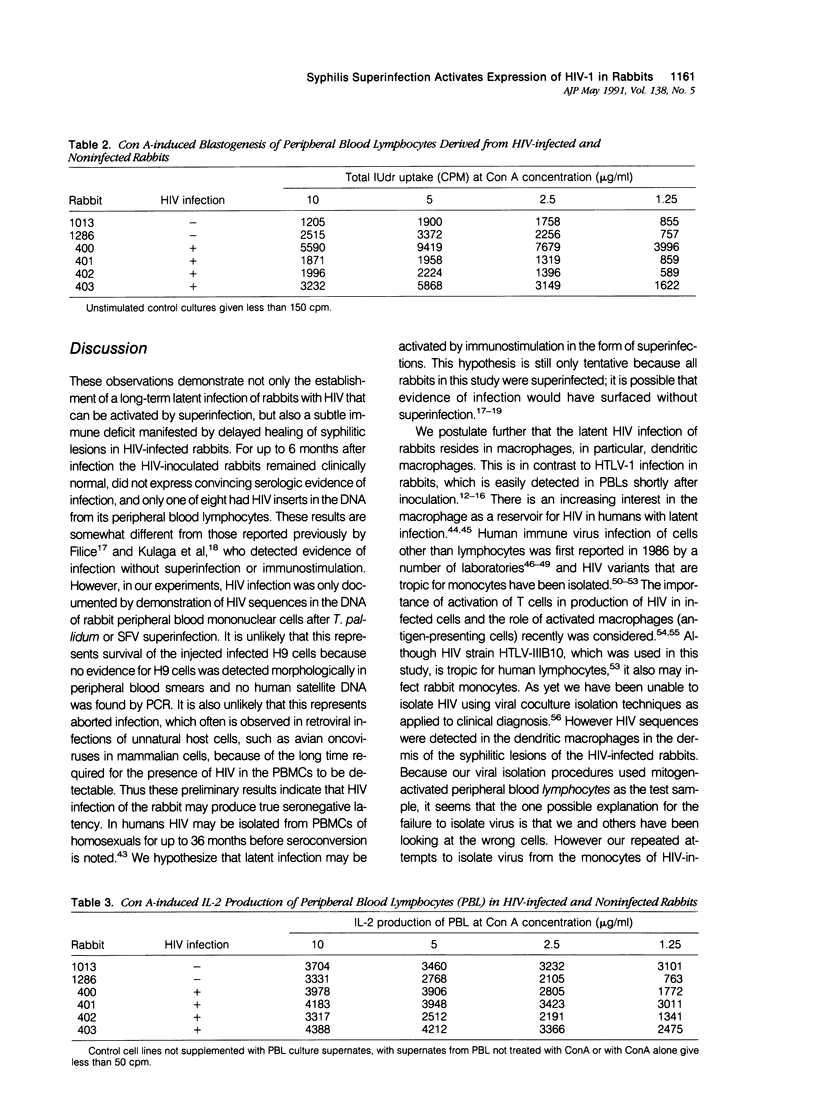
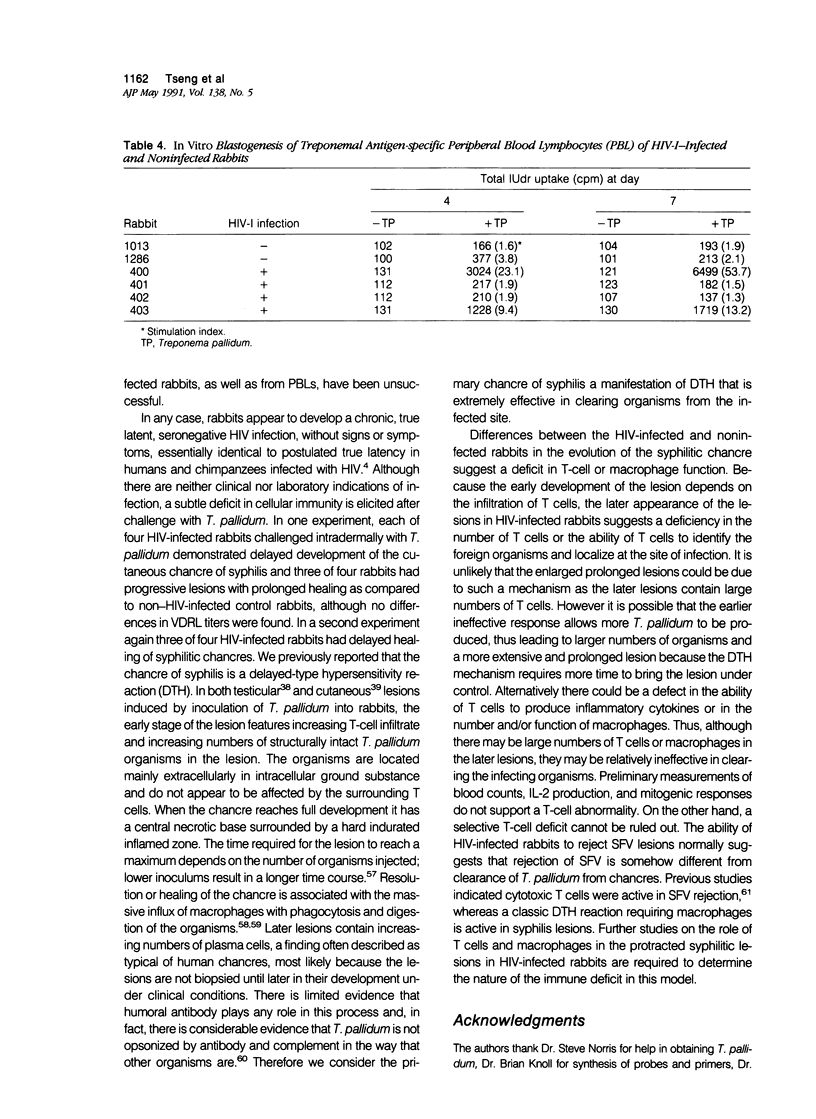


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi T., Takeda I., Oka T., Ohtsuki Y., Yano S., Miyoshi I. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn J Cancer Res. 1985 Feb;76(2):86–94. [PubMed] [Google Scholar]
- Alter H. J., Eichberg J. W., Masur H., Saxinger W. C., Gallo R., Macher A. M., Lane H. C., Fauci A. S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984 Nov 2;226(4674):549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Ascher M. S., Sheppard H. W. AIDS as immune system activation: a model for pathogenesis. Clin Exp Immunol. 1988 Aug;73(2):165–167. [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P. Natural immunity to HIV and its possible relationship to vaccine strategies. Microbiol Sci. 1988 Aug;5(8):236–241. [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- De Koning J., Bosma R. B., Hoogkamp-Korstanje J. A. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J Med Microbiol. 1987 May;23(3):261–267. doi: 10.1099/00222615-23-3-261. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. Simian immunodeficiency viruses. Annu Rev Microbiol. 1988;42:607–625. doi: 10.1146/annurev.mi.42.100188.003135. [DOI] [PubMed] [Google Scholar]
- Filice G., Cereda P. M., Varnier O. E. Infection of rabbits with human immunodeficiency virus. Nature. 1988 Sep 22;335(6188):366–369. doi: 10.1038/335366a0. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Petricciani J. C. The prospects for and pathways toward a vaccine for AIDS. N Engl J Med. 1985 Dec 19;313(25):1586–1590. doi: 10.1056/NEJM198512193132506. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Luciw P. A. Animal models of AIDS. FASEB J. 1989 Dec;3(14):2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa D. T., Lee M. H., Wolinsky S. M., Sano K., Morales F., Kwok S., Sninsky J. J., Nishanian P. G., Giorgi J., Fahey J. L. Human immunodeficiency virus type 1 infection in homosexual men who remain seronegative for prolonged periods. N Engl J Med. 1989 Jun 1;320(22):1458–1462. doi: 10.1056/NEJM198906013202205. [DOI] [PubMed] [Google Scholar]
- Izzat N. N., Knox J. M., Werth J. A., Dacres W. G. Evolution of syphilitic chancres with virulent Treponema pallidum in the rabbit. Br J Vener Dis. 1971 Apr;47(2):67–72. doi: 10.1136/sti.47.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel-Reid S., Dick J. E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988 Dec 23;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kulaga H., Folks T., Rutledge R., Truckenmiller M. E., Gugel E., Kindt T. J. Infection of rabbits with human immunodeficiency virus 1. A small animal model for acquired immunodeficiency syndrome. J Exp Med. 1989 Jan 1;169(1):321–326. doi: 10.1084/jem.169.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. M., Abramczuk J. W., Pezen D. S., Rutledge R., Belcher J. H., Hakim F., Shearer G., Lamperth L., Travis W., Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988 Dec 23;242(4886):1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Pruett J. E., Cohn Z. A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci U S A. 1989 Jan;86(2):675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Yoshimoto S., Taguchi H., Kubonishi I., Fujishita M., Ohtsuki Y., Shiraishi Y., Akagi T. Transformation of rabbit lymphocytes with T-cell leukemia virus. Gan. 1983 Feb;74(1):1–4. [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Davison-Fairburn B., Montelaro R. C., Miller M., West M., Ohkawa S., Baskin G. B., Zhang J. Y., Putney S. D. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989 Dec 8;246(4935):1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Ogawa K., Matsuda S., Seto A. Induction of leukemic infiltration by allogeneic transfer of HTLV-I-transformed T cells in rabbits. Leuk Res. 1989;13(5):399–406. doi: 10.1016/0145-2126(89)90080-5. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pauza C. D. HIV persistence in monocytes leads to pathogenesis and AIDS. Cell Immunol. 1988 Apr 1;112(2):414–424. doi: 10.1016/0008-8749(88)90310-3. [DOI] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Gartner S. Isolation of HIV-1 from monocytes but not T lymphocytes. Lancet. 1987 Oct 17;2(8564):916–916. doi: 10.1016/s0140-6736(87)91403-6. [DOI] [PubMed] [Google Scholar]
- Rentrop M., Knapp B., Winter H., Schweizer J. Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986 May;18(5):271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Schneider J., Hunsmann G. Simian lentiviruses--the SIV group. AIDS. 1988 Feb;2(1):1–9. doi: 10.1097/00002030-198802000-00001. [DOI] [PubMed] [Google Scholar]
- Scott C. B., Holdbrook R., Sell S. Cell-mediated immune response to Shope fibroma virus-induced tumors in adult rabbits. J Natl Cancer Inst. 1981 Apr;66(4):681–689. [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Sell S., Norris S. J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- Sell S., Scott C. B. An immunohistologic study of Shope fibroma virus in rabbits: tumor rejection by cellular reaction in adults and progressive systemic reticuloendothelial infection in neonates. J Natl Cancer Inst. 1981 Feb;66(2):363–373. [PubMed] [Google Scholar]
- Seto A., Kawanishi M., Matsuda S., Ogawa K. Seronegative virus carriers in the infection of rabbits with human T lymphotropic virus type I. J Exp Med. 1988 Dec 1;168(6):2409–2414. doi: 10.1084/jem.168.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer D. S., Sell S. Immunohistology of malignant rabbit fibroma virus--a comparative study with rabbit myxoma virus. J Natl Cancer Inst. 1983 Jul;71(1):105–116. [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Sell S. Strain differences in Shope fibroma virus. An immunopathologic study. Am J Pathol. 1984 Aug;116(2):342–358. [PMC free article] [PubMed] [Google Scholar]
- Truckenmiller M. E., Kulaga H., Gugel E., Dickerson D., Kindt T. J. Evidence for dual infection of rabbits with the human retroviruses HTLV-I and HIV-1. Res Immunol. 1989 Jun-Aug;140(5-6):527–544. doi: 10.1016/0923-2494(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Darling S. M., Erickson R. P., Craig I. W., Buckle V. J., Rigby P. W., Willard H. F., Goodfellow P. N. Isolation and characterization of an alphoid centromeric repeat family from the human Y chromosome. J Mol Biol. 1985 Apr 20;182(4):477–485. doi: 10.1016/0022-2836(85)90234-7. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]