Abstract
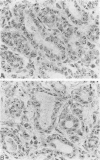
The argyrophilic staining of the nucleolar organizer region (AgNOR) in cells of Dunning R3327 rat prostate tumors was studied and the effect of hormonal treatments on their appearance was analyzed. The nuclei of the control tumor cells contained 4.1 +/- 0.17 AgNOR granules. Treatment of rats for 8 weeks with luteinizing hormone-releasing hormone (LH-RH) agonist (D-Trp-6-LH-RH) and antagonist SB-75 induced a marked inhibition of tumor growth and decreased significantly (P less than 0.01) the number of Ag-NORs in the tumors to 2.89 +/- 0.10 AgNOR granules/cell in the group given the agonist and to 2.82 +/- 0.10 after therapy with the highest dose of the antagonist. A reduced AgNOR number (3.14 +/- 0.16) also was found after 3 days of treatment with SB-75 (P less than 0.05), but the AgNORs returned to near control values 1 week after the short-term therapy, showing the reversibility of these changes. These results suggest that the AgNOR method, which was widely tested on human tumors in the past few years, can be a valuable technique in experimental tumor pathology and useful in the evaluation of the effects of various treatments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz S., Csernus V. J., Janaky T., Bokser L., Fekete M., Schally A. V. New antagonists of LHRH. II. Inhibition and potentiation of LHRH by closely related analogues. Int J Pept Protein Res. 1988 Dec;32(6):425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Chiu K. Y., Loke S. L., Wong K. K. Improved silver technique for showing nucleolar organiser regions in paraffin wax sections. J Clin Pathol. 1989 Sep;42(9):992–994. doi: 10.1136/jcp.42.9.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M., Pession A., Farabegoli F., Trerè D., Badiali M., Dehan P. Relationship between interphasic nucleolar organizer regions and growth rate in two neuroblastoma cell lines. Am J Pathol. 1989 Apr;134(4):925–932. [PMC free article] [PubMed] [Google Scholar]
- Dervan P. A., Gilmartin L. G., Loftus B. M., Carney D. N. Breast carcinoma kinetics. Argyrophilic nucleolar organizer region counts correlate with Ki67 scores. Am J Clin Pathol. 1989 Oct;92(4):401–407. doi: 10.1093/ajcp/92.4.401. [DOI] [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- Korkut E., Bokser L., Comaru-Schally A. M., Groot K., Schally A. V. Inhibition of growth of experimental prostate cancer with sustained delivery systems (microcapsules and microgranules) of the luteinizing hormone-releasing hormone antagonist SB-75. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):844–848. doi: 10.1073/pnas.88.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles S. E., McNicol A. M. AgNOR numbers in rat pituitary corticotrophs following adrenalectomy or corticotrophin releasing factor administration. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):209–212. doi: 10.1007/BF02899083. [DOI] [PubMed] [Google Scholar]
- Quinn C. M., Wright N. A. The clinical assessment of proliferation and growth in human tumours: evaluation of methods and applications as prognostic variables. J Pathol. 1990 Feb;160(2):93–102. doi: 10.1002/path.1711600202. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Redding T. W. Combination of long-acting microcapsules of the D-tryptophan-6 analog of luteinizing hormone-releasing hormone with chemotherapy: investigation in the rat prostate cancer model. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2498–2502. doi: 10.1073/pnas.82.8.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V., Redding T. W. Somatostatin analogs as adjuncts to agonists of luteinizing hormone-releasing hormone in the treatment of experimental prostate cancer. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7275–7279. doi: 10.1073/pnas.84.20.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Ochs R. L., Busch H. Silver staining, immunofluorescence, and immunoelectron microscopic localization of nucleolar phosphoproteins B23 and C23. Chromosoma. 1984;90(2):139–148. doi: 10.1007/BF00292451. [DOI] [PubMed] [Google Scholar]
- Suresh U. R., Chawner L., Buckley C. H., Fox H. Do AgNOR counts reflect cellular ploidy or cellular proliferation? A study of trophoblastic tissue. J Pathol. 1990 Mar;160(3):213–215. doi: 10.1002/path.1711600306. [DOI] [PubMed] [Google Scholar]
- Tham K. T., Page D. L. AgNOR and Ki-67 in breast lesions. Am J Clin Pathol. 1989 Oct;92(4):518–520. doi: 10.1093/ajcp/92.4.518. [DOI] [PubMed] [Google Scholar]
- Trerè D., Pession A., Derenzini M. The silver-stained proteins of interphasic nucleolar organizer regions as a parameter of cell duplication rate. Exp Cell Res. 1989 Sep;184(1):131–137. doi: 10.1016/0014-4827(89)90371-6. [DOI] [PubMed] [Google Scholar]
- Wake N., Isaacs J., Sandberg A. A. Chromosomal changes associated with progression of the Dunning R-3327 rat prostatic adenocarcinoma system. Cancer Res. 1982 Oct;42(10):4131–4142. [PubMed] [Google Scholar]
- Yan Y. S., Stanley W. S. Effect of differentiating agents on nucleolar organizer region activity in human melanoma cells. Cancer Genet Cytogenet. 1988 Apr;31(2):253–262. doi: 10.1016/0165-4608(88)90225-7. [DOI] [PubMed] [Google Scholar]
- Zalatnai A., Paz-Bouza J. I., Redding T. W., Schally A. V. Histologic changes in the rat prostate cancer model after treatment with somatostatin analogs and D-Trp-6-LH-RH. Prostate. 1988;12(1):85–98. doi: 10.1002/pros.2990120111. [DOI] [PubMed] [Google Scholar]
- de Capoa A., Baldini A., Marlekaj P., Natoli C., Rocchi M., Archidiacono N., Cianfarani S., Spadoni G. L., Boscherini B. Hormone-modulated rRNA gene activity is visualized by selective staining of the NOs. Cell Biol Int Rep. 1985 Sep;9(9):791–796. doi: 10.1016/0309-1651(85)90097-9. [DOI] [PubMed] [Google Scholar]