Abstract
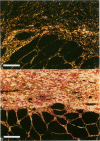
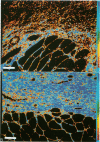
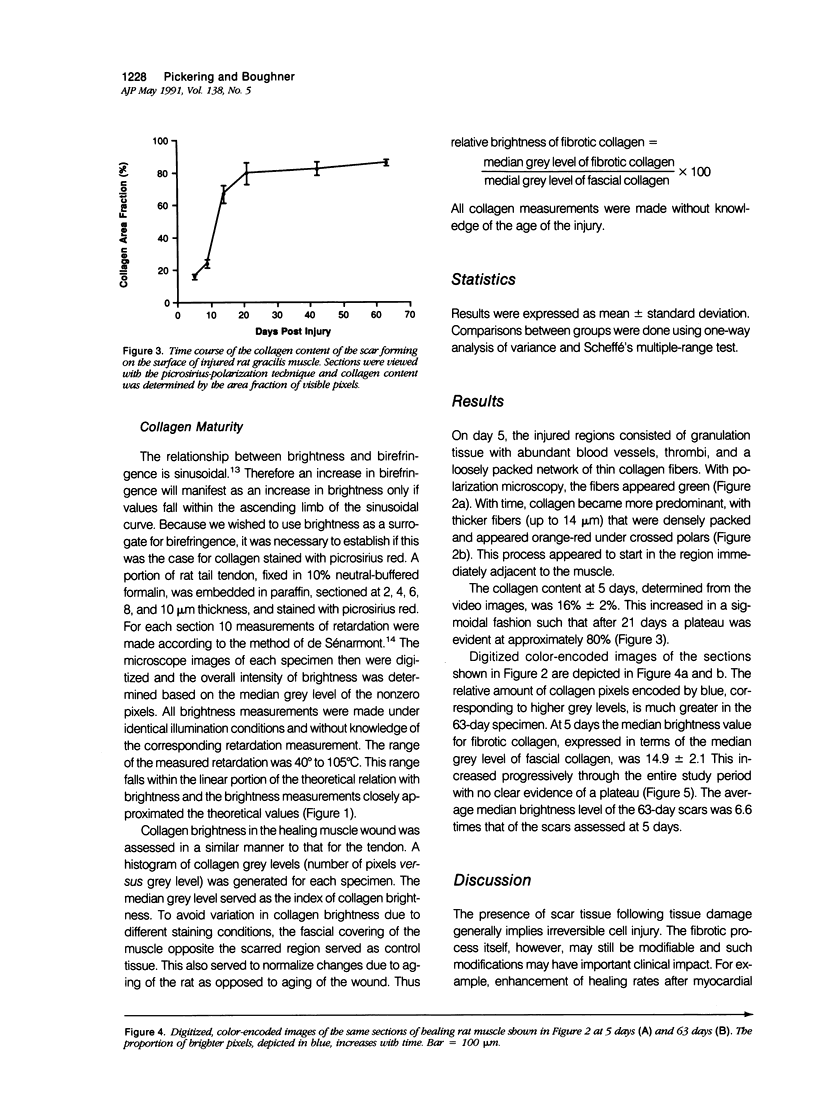
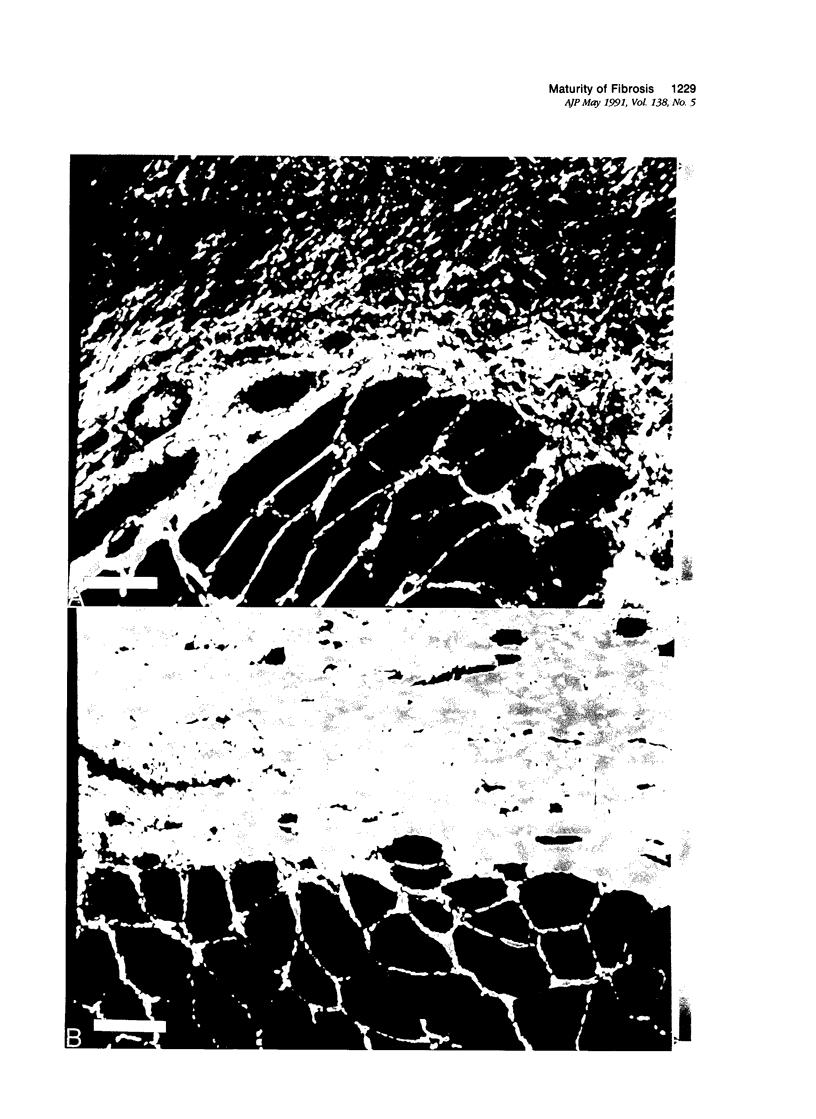
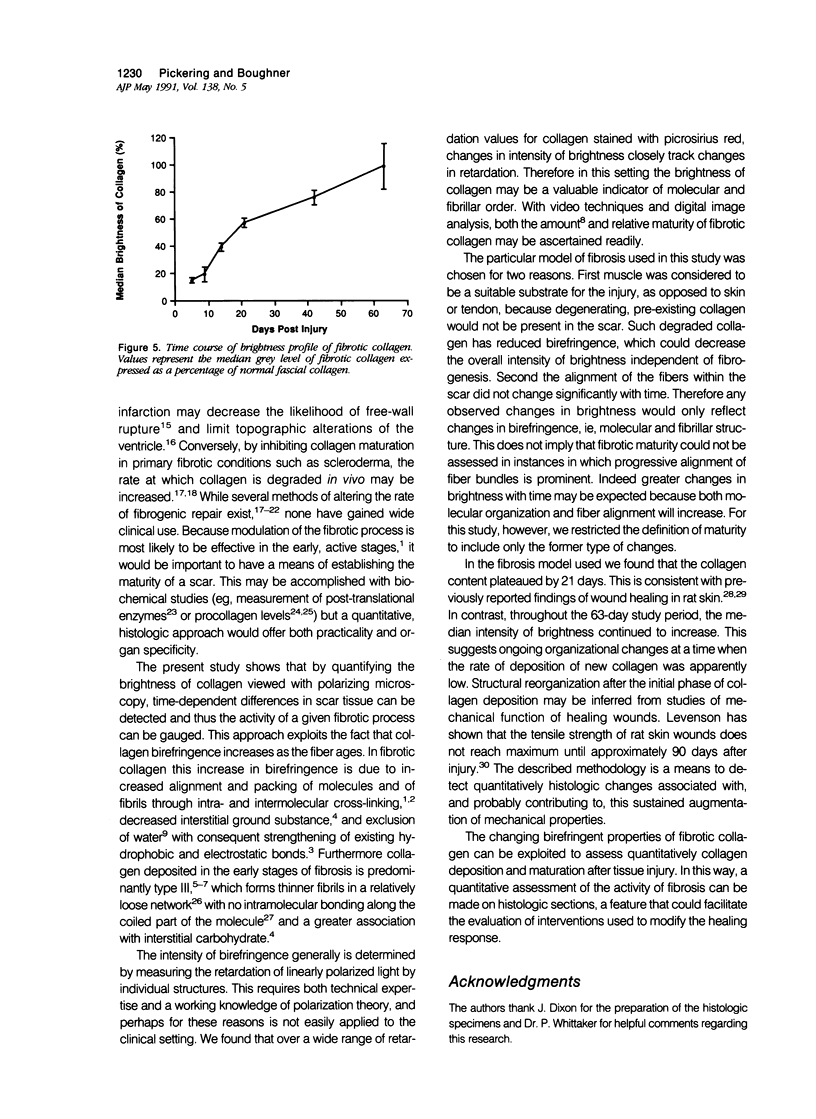
Reliable histologic methods for gauging the maturity of fibrotic lesions are limited, making interventions in the healing process difficult to assess. As collagen ages there is enhanced birefringence due to increased molecular and fibrillar organization. The purpose of this study was to develop a microscopal technique to quantify this process and to determine its ability to distinguish scars of varying ages. Fibrosis in the rat gracilis muscle was studied 5 to 63 days after superficial injury. Sections were stained with picrosirius red and illuminated with monochromatic, polarized light. The microscope fields were digitized using a computer-video system yielding an image in which noncollagenous material was dark (gray level 0) and collagen was depicted by grey levels 1 to 255. In the fibrosis model used, the collagen area fraction plateaued at 80% by day 21. The median collagen grey level increased progressively as the scar aged. It is concluded that this histologic, nondestructive technique can reliably quantify age-related optical properties of fibrotic collagen and that this could be used to determine the maturity of fibrotic lesions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Light N. D. Intermolecular cross-linking in fibrotic collagen. Ciba Found Symp. 1985;114:80–96. doi: 10.1002/9780470720950.ch6. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Morton L. F., Bennett R. C., Bailey A. J., Sims T. J. Presence of type III collagen in guinea-pig dermal scar. Biochem J. 1976 Jul 1;157(1):263–266. doi: 10.1042/bj1570263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- Clore J. N., Cohen I. K., Diegelmann R. F. Quantitation of collagen types I and III during wound healing in rat skin. Proc Soc Exp Biol Med. 1979 Jul;161(3):337–340. doi: 10.3181/00379727-161-40548. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., Moore C. D., Diegelmann R. F. Onset and localization of collagen synthesis during wound healing in open rat skin wounds. Proc Soc Exp Biol Med. 1979 Apr;160(4):458–462. doi: 10.3181/00379727-160-40470. [DOI] [PubMed] [Google Scholar]
- Constantine V. S., Mowry R. W. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968 May;50(5):419–423. doi: 10.1038/jid.1968.68. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Sjoerdsma A. Effect of penicillamine on human collagen and its possible application to treatment of scleroderma. Lancet. 1966 Nov 5;2(7471):996–999. doi: 10.1016/s0140-6736(66)92926-6. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Voss T., Kühn K., Engel J. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol. 1984 Jan 25;172(3):325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- Jensen L. T., Hørslev-Petersen K., Toft P., Bentsen K. D., Grande P., Simonsen E. E., Lorenzen I. Serum aminoterminal type III procollagen peptide reflects repair after acute myocardial infarction. Circulation. 1990 Jan;81(1):52–57. doi: 10.1161/01.cir.81.1.52. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Cossermelli W., Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978 Jun;41(3):267–274. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- LEVENSON S. M., GEEVER E. F., CROWLEY L. V., OATES J. F., 3rd, BERARD C. W., ROSEN H. THE HEALING OF RAT SKIN WOUNDS. Ann Surg. 1965 Feb;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman R. H., Apstein C. S., Kagan H. M., Osmers E. L., Chichester C. O., Vogel W. M., Connelly C. M., Steffee W. P. Myocardial healing and repair after experimental infarction in the rabbit. Circ Res. 1983 Sep;53(3):378–388. doi: 10.1161/01.res.53.3.378. [DOI] [PubMed] [Google Scholar]
- Madden J. W., Davis W. M., Butler C., 2nd, Peacock E. E., Jr Experimental esophageal lye burns. II. Correcting established strictures with beta-aminopropionitrile and bougienage. Ann Surg. 1973 Sep;178(3):277–284. doi: 10.1097/00000658-197309000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. W., Peacock E. E., Jr Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery. 1968 Jul;64(1):288–294. [PubMed] [Google Scholar]
- Nimni M. E. The molecular organization of collagen and its role in determining the biophysical properties of the connective tissues. Biorheology. 1980;17(1-2):51–82. doi: 10.3233/bir-1980-171-210. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990 Apr;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Pickering J. G., Boughner D. R. Fibrosis in the transplanted heart and its relation to donor ischemic time. Assessment with polarized light microscopy and digital image analysis. Circulation. 1990 Mar;81(3):949–958. doi: 10.1161/01.cir.81.3.949. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Williams I. F., McCullagh K. G., Silver I. A. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12(3-4):211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]
- Wolman M. Polarized light microscopy as a tool of diagnostic pathology. J Histochem Cytochem. 1975 Jan;23(1):21–50. doi: 10.1177/23.1.1090645. [DOI] [PubMed] [Google Scholar]