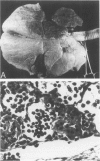
Abstract
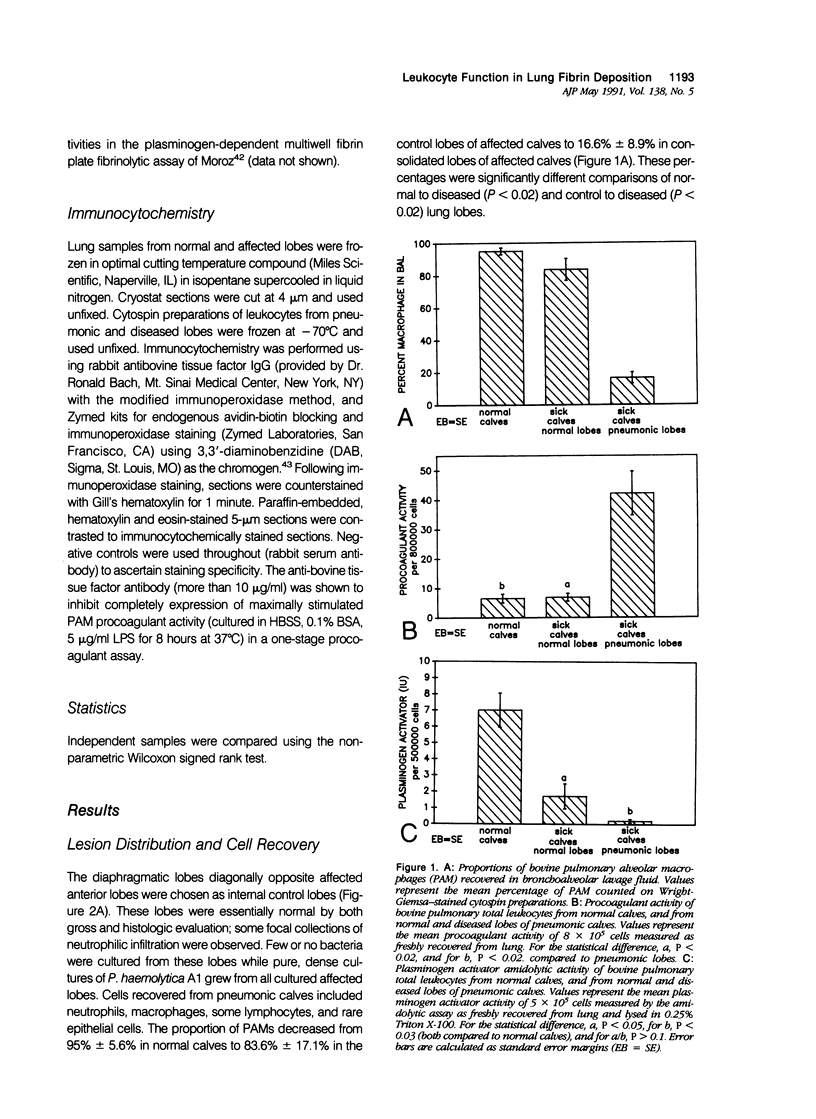
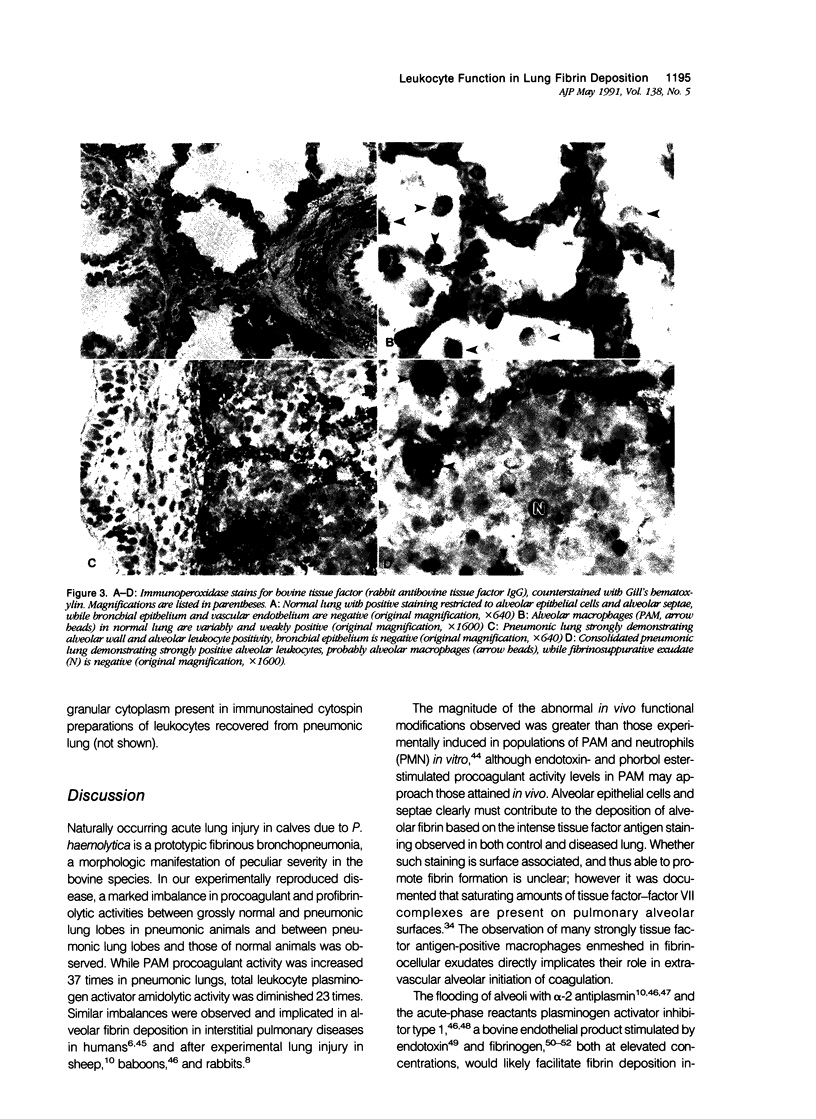
The peculiarly fibrinous nature of bovine acute lung injury due to infection with Pasteurella haemolytica A1 suggests an imbalance between leukocyte-directed procoagulant and profibrinolytic influences in the inflamed bovine lung. Calves with experimental pneumonia produced by intratracheal inoculation with P. haemolytica A1 developed acute locally extensive cranioventral fibrinopurulent bronchopneumonia. Pulmonary alveolar macrophages (PAM) recovered by segmental lavage from affected lung lobes were 30 times more procoagulant than PAM obtained from unaffected lung lobes and 37-fold more procoagulant than PAM from control calf lungs. Unlike the enhancement of procoagulant activity, profibrinolytic activity (plasminogen activator amidolysis) of total lung leukocytes (PAM and plasminogen activator neutrophils [PMN]) was decreased 23 times in cells obtained from affected lung lobes and also was decreased four times in cells obtained from unaffected lobes of infected animals. This marked imbalance in cellular procoagulant and fibrinolytic activity probably contributes significantly to enhanced fibrin deposition and retarded fibrin removal. In addition, PAM from inflamed lungs were strongly positive for bovine tissue factor antigen as demonstrated by immunocytochemistry. Intensely tissue factor-positive PAM enmeshed in fibrinocellular exudates and positive alveolar walls were situated such that they were likely to have, in concert, initiated extrinsic activation of coagulation in the acutely inflamed lung. These data collectively suggest that enhanced PAM-directed procoagulant activity and diminished PAM- and PMN-directed profibrinolytic activity represent important modifications of local leukocyte function in bovine acute lung injury that are central to the pathogenesis of lesion development with extensive fibrin deposition and retarded fibrin removal.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Young L., Bowden D. H. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988 Feb;130(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- Basset F., Ferrans V. J., Soler P., Takemura T., Fukuda Y., Crystal R. G. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986 Mar;122(3):443–461. [PMC free article] [PubMed] [Google Scholar]
- Bowen R. M., Hoidal J. R., Estensen R. D. Urokinase-type plasminogen activator in alveolar macrophages and bronchoalveolar lavage fluid from normal and smoke-exposed hamsters and humans. J Lab Clin Med. 1985 Dec;106(6):667–673. [PubMed] [Google Scholar]
- Bretscher M. S., Bretcher M. S. Fibroblasts on the move. J Cell Biol. 1988 Feb;106(2):235–237. doi: 10.1083/jcb.106.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin G., Einarsson M., Saldeen T. Delayed elimination of fibrin from the lungs in rats given alpha 2-antiplasmin. Thromb Haemost. 1981 Dec 23;46(4):757–758. [PubMed] [Google Scholar]
- Chapman H. A., Allen C. L., Stone O. L. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986 Mar;133(3):437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Allen C. L., Stone O. L., Fair D. S. Human alveolar macrophages synthesize factor VII in vitro. Possible role in interstitial lung disease. J Clin Invest. 1985 Jun;75(6):2030–2037. doi: 10.1172/JCI111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Reilly J. J., Jr, Kobzik L. Role of plasminogen activator in degradation of extracellular matrix protein by live human alveolar macrophages. Am Rev Respir Dis. 1988 Feb;137(2):412–419. doi: 10.1164/ajrccm/137.2.412. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Stahl M., Allen C. L., Yee R., Fair D. S. Regulation of the procoagulant activity within the bronchoalveolar compartment of normal human lung. Am Rev Respir Dis. 1988 Jun;137(6):1417–1425. doi: 10.1164/ajrccm/137.6.1417. [DOI] [PubMed] [Google Scholar]
- Christman J. W., Petras S. F., Hacker M., Absher P. M., Davis G. S. Alveolar macrophage function is selectively altered after endotoxemia in rats. Infect Immun. 1988 May;56(5):1254–1259. doi: 10.1128/iai.56.5.1254-1259.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeson G., Friberger P., Knös M., Eriksson E. Methods for determination of prekallikrein in plasma, glandular kallikrein and urokinase. Haemostasis. 1978;7(2-3):76–78. doi: 10.1159/000214238. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Greenberg J. Modulation of the effects of alveolar macrophages on lung fibroblast collagen production rate. Am Rev Respir Dis. 1987 Jan;135(1):52–56. doi: 10.1164/arrd.1987.135.1.52. [DOI] [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B. Fibrinogen-fibrin interactions with fibroblasts and macrophages. Ann N Y Acad Sci. 1983 Jun 27;408:621–633. doi: 10.1111/j.1749-6632.1983.tb23279.x. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Conanan L. B., Ryan U. S. Endotoxin-induced secretion of an active plasminogen activator inhibitor from bovine pulmonary arterial and aortic endothelial cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1346–1353. doi: 10.1016/s0006-291x(87)80280-2. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Colditz I. G., Movat H. Z. The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol. 1986 Sep;124(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Denholm E. M., Phan S. H. The effects of bleomycin on alveolar macrophage growth factor secretion. Am J Pathol. 1989 Feb;134(2):355–363. [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Morrissey J. H., Edgington T. S. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989 May;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Eckersall P. D., Conner J. G. Bovine and canine acute phase proteins. Vet Res Commun. 1988;12(2-3):169–178. doi: 10.1007/BF00362798. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Krol R. C., Freundlich B., Sampson P. M. Regulation of human lung fibroblast glycosaminoglycan production by recombinant interferons, tumor necrosis factor, and lymphotoxin. J Clin Invest. 1988 Feb;81(2):325–333. doi: 10.1172/JCI113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. C., Thomson R. G., Wilkie B. N. Pulmonary lesions induced by Pasteurella hemolytica in cattle. Can J Comp Med. 1977 Apr;41(2):219–223. [PMC free article] [PubMed] [Google Scholar]
- Geczy C. L., Meyer P. A. Leukocyte procoagulant activity in man: an in vitro correlate of delayed-type hypersensitivity. J Immunol. 1982 Jan;128(1):331–336. [PubMed] [Google Scholar]
- Gibbs H. A., Allan E. M., Wiseman A., Selman I. E. Experimental production of bovine pneumonic pasteurellosis. Res Vet Sci. 1984 Sep;37(2):154–166. [PubMed] [Google Scholar]
- Grünig G., Hermann M., Winder C., Von Fellenberg R. Procoagulant activity in respiratory tract secretions from horses with chronic pulmonary disease. Am J Vet Res. 1988 May;49(5):705–709. [PubMed] [Google Scholar]
- Haritani M., Nakazawa M., Oohashi S., Yamada Y., Haziroglu R., Narita M. Immunoperoxidase evaluation of pneumonic lesions induced by Pasteurella haemolytica in calves. Am J Vet Res. 1987 Sep;48(9):1358–1362. [PubMed] [Google Scholar]
- Hasday J. D., Bachwich P. R., Lynch J. P., 3rd, Sitrin R. G. Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis. Exp Lung Res. 1988;14(2):261–278. doi: 10.3109/01902148809115128. [DOI] [PubMed] [Google Scholar]
- Hawkey C. M., Hart M. G. Fibrinogen levels in mammals suffering from bacterial infections. Vet Rec. 1987 Nov 28;121(22):519–521. doi: 10.1136/vr.121.22.519. [DOI] [PubMed] [Google Scholar]
- Heiple J. M., Ossowski L. Human neutrophil plasminogen activator is localized in specific granules and is translocated to the cell surface by exocytosis. J Exp Med. 1986 Sep 1;164(3):826–840. doi: 10.1084/jem.164.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S., Gonzalez K. K., MacArthur C. K., Gillies C., Walsh P. N., McLarty J., Thrall R. S. Bronchoalveolar lavage procoagulant activity in bleomycin-induced lung injury in marmosets. Characterization and relationship to fibrin deposition and fibrosis. Am Rev Respir Dis. 1987 Jul;136(1):124–133. doi: 10.1164/ajrccm/136.1.124. [DOI] [PubMed] [Google Scholar]
- Idell S., Gonzalez K., Bradford H., MacArthur C. K., Fein A. M., Maunder R. J., Garcia J. G., Griffith D. E., Weiland J., Martin T. R. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis. 1987 Dec;136(6):1466–1474. doi: 10.1164/ajrccm/136.6.1466. [DOI] [PubMed] [Google Scholar]
- Idell S., Peters J., James K. K., Fair D. S., Coalson J. J. Local abnormalities of coagulation and fibrinolytic pathways that promote alveolar fibrin deposition in the lungs of baboons with diffuse alveolar damage. J Clin Invest. 1989 Jul;84(1):181–193. doi: 10.1172/JCI114139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S., Peterson B. T., Gonzalez K. K., Gray L. D., Bach R., McLarty J., Fair D. S. Local abnormalities of coagulation and fibrinolysis and alveolar fibrin deposition in sheep with oleic acid-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1282–1294. doi: 10.1164/ajrccm/138.5.1282. [DOI] [PubMed] [Google Scholar]
- Janson T. L., Stormorken H., Prydz H. Species specificity of tissue thromboplastin. Haemostasis. 1984;14(5):440–444. doi: 10.1159/000215102. [DOI] [PubMed] [Google Scholar]
- Jordana M., Newhouse M. T., Gauldie J. Alveolar macrophage/peripheral blood monocyte-derived factors modulate proliferation of primary lines of human lung fibroblasts. J Leukoc Biol. 1987 Jul;42(1):51–60. doi: 10.1002/jlb.42.1.51. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Gudinchet A., Bachmann F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thromb Haemost. 1988 Feb 25;59(1):7–12. [PubMed] [Google Scholar]
- Kumar R. K., Bennett R. A., Brody A. R. A homologue of platelet-derived growth factor produced by rat alveolar macrophages. FASEB J. 1988 Apr;2(7):2272–2277. doi: 10.1096/fasebj.2.7.3280379. [DOI] [PubMed] [Google Scholar]
- Lay J. C., Slauson D. O., Castleman W. L. Volume-controlled bronchopulmonary lavage of normal and pneumonic calves. Vet Pathol. 1986 Nov;23(6):673–680. doi: 10.1177/030098588602300605. [DOI] [PubMed] [Google Scholar]
- Lemaire I., Beaudoin H., Massé S., Grondin C. Alveolar macrophage stimulation of lung fibroblast growth in asbestos-induced pulmonary fibrosis. Am J Pathol. 1986 Feb;122(2):205–211. [PMC free article] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Maxie M. G., Valli V. E., Robinson G. A., Truscott R. B., McSherry B. J. Studies with radioactive endotoxin. I. Clearance of 51Cr-labelled endotoxin from the blood of calves. Can J Comp Med. 1974 Oct;38(4):347–366. [PMC free article] [PubMed] [Google Scholar]
- McSherry B. J., Horney F. D., DeGroot J. J. Plasma fibrinogen levels in normal and sick cows. Can J Comp Med. 1970 Jul;34(3):191–197. [PMC free article] [PubMed] [Google Scholar]
- Moroz L. A., Gilmore N. J. A rapid and sensitive 125I-fibrin solid-phase fibrinolytic assay for plasmin. Blood. 1975 Oct;46(4):543–553. [PubMed] [Google Scholar]
- Newton R. C. Human monocyte production of interleukin-1: parameters of the induction of interleukin-1 secretion by lipopolysaccharides. J Leukoc Biol. 1986 Mar;39(3):299–311. doi: 10.1002/jlb.39.3.299. [DOI] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberger H., McGee M. P., Lee T. K. Tissue factor activity. A marker of alveolar macrophage maturation in rabbits. Effects of granulomatous pneumonitis. J Clin Invest. 1984 Jun;73(6):1524–1531. doi: 10.1172/JCI111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Hovi T., Vaheri A. Urokinase-type plasminogen activator and its inhibitor secreted by cultured human monocyte-macrophages. J Cell Physiol. 1985 Jan;122(1):125–132. doi: 10.1002/jcp.1041220119. [DOI] [PubMed] [Google Scholar]
- Schwartz B. S., Monroe M. C., Levin E. G. Increased release of plasminogen activator inhibitor type 2 accompanies the human mononuclear cell tissue factor response to lipopolysaccharide. Blood. 1988 Mar;71(3):734–741. [PubMed] [Google Scholar]
- Sitrin R. G., Brubaker P. G., Fantone J. C. Tissue fibrin deposition during acute lung injury in rabbits and its relationship to local expression of procoagulant and fibrinolytic activities. Am Rev Respir Dis. 1987 Apr;135(4):930–936. doi: 10.1164/arrd.1987.135.4.930. [DOI] [PubMed] [Google Scholar]
- Slauson D. O. The mediation of pulmonary inflammatory injury. Adv Vet Sci Comp Med. 1982;26:99–153. [PubMed] [Google Scholar]
- Tabor D. R., Burchett S. K., Jacobs R. F. Enhanced production of monokines by canine alveolar macrophages in response to endotoxin-induced shock. Proc Soc Exp Biol Med. 1988 Apr;187(4):408–415. doi: 10.3181/00379727-187-42681. [DOI] [PubMed] [Google Scholar]
- Turck C. W., Dohlman J. G., Goetzl E. J. Immunological mediators of wound healing and fibrosis. J Cell Physiol Suppl. 1987;Suppl 5:89–93. doi: 10.1002/jcp.1041330417. [DOI] [PubMed] [Google Scholar]
- Weissler J. C. Idiopathic pulmonary fibrosis: cellular and molecular pathogenesis. Am J Med Sci. 1989 Feb;297(2):91–104. doi: 10.1097/00000441-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Zwahlen R. D., Slauson D. O., Neilsen N. R., Clifford C. B. Increased adhesiveness of complement-stimulated neonatal calf neutrophils and its pharmacologic inhibition. J Leukoc Biol. 1987 Jun;41(6):465–473. doi: 10.1002/jlb.41.6.465. [DOI] [PubMed] [Google Scholar]