Abstract
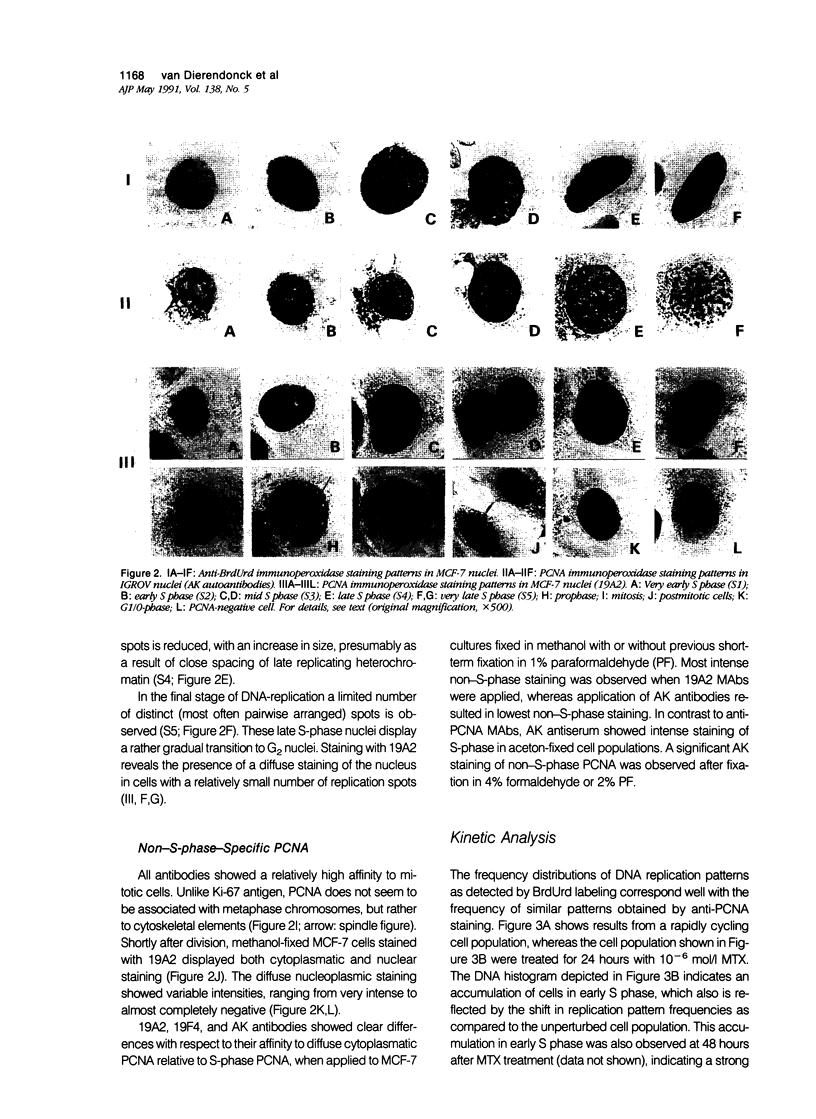
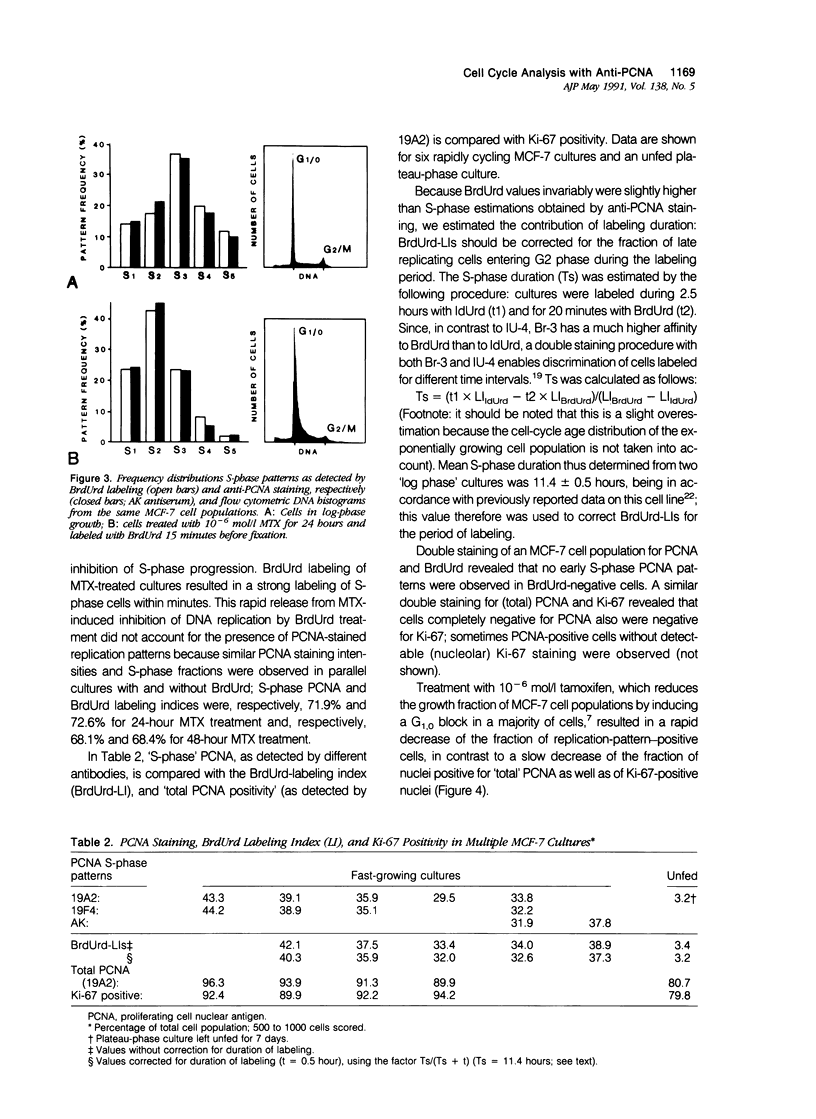
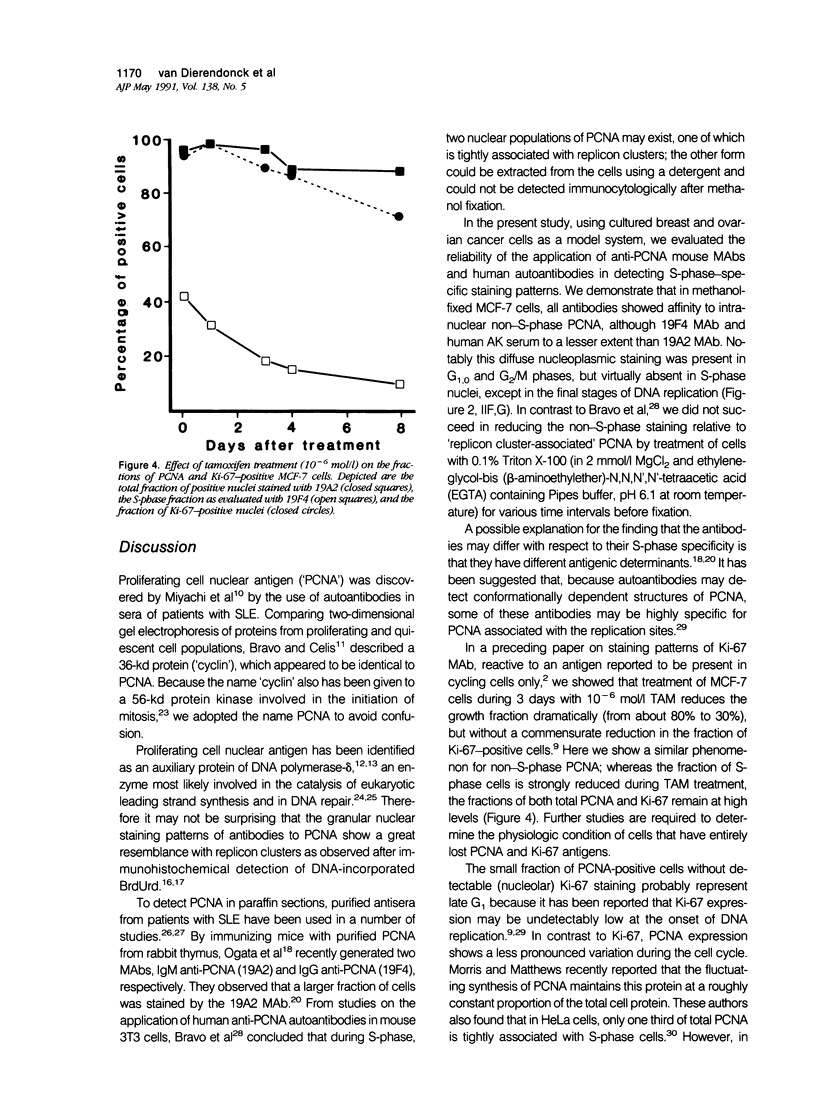
Monoclonal antibodies (MAbs) to nuclear antigens are increasingly used as tools to obtain valuable information concerning the proliferative characteristics of various types of cancer. Prerequisite for the application of these MAbs in surgical pathology is establishment of the level of expression and/or cellular distribution of the antigens in relation to distinct cell-cycle compartments. In this study the topologic distribution of proliferating cell nuclear antigen (PCNA), an auxiliary protein of DNA polymerase delta, as recognized by human autoantiserum (AK) and two recently developed MAbs (19A2 and 19F4), was evaluated. Using cultured human cancer cells as a model system, and providing optimal fixation/permeation procedures are applied, these antibodies display a high affinity for PCNA bound to nuclear replicon clusters, resulting in distinct granular staining patterns. A more diffuse nucleoplasmic PCNA staining was mainly restricted to non-S-phase cells; in methanol-fixed cells, staining intensity of this form relative to the replicon-bound form appeared higher after staining with 19A2 than with 19F4 or AK. Comparing PCNA expression (detected with 19A2) with the expression of the Ki-67 antigen, PCNA-negative cells are also Ki-67 negative. In MCF-7 human breast cancer cells treated with 10(-6) mol/l (molar) tamoxifen, the fraction of nuclei showing replication patterns decreased from 42% to 8% within 8 days, but PCNA and Ki-67 antigens remained detectable in most cells during this interval, indicating a relatively slow decrease of antigen expression in cells that have entered a quiescent state. Treatment of MCF-7 cells with 10(-6) mol/l methotrexate resulted in a rapid accumulation of cells with an early S-phase DNA content; PCNA replication patterns showing a frequency distribution reflecting this DNA content were observed up to 48 hours after treatment. This indicates that the presence of replication patterns as visualized with anti-PCNAs is not a measure of replicative activity per se. It is concluded that, providing nuclear non-S-phase PCNA staining is faint relative to staining of replicon clusters, anti-PCNA antibodies may be excellent markers to detect in situ cells with S-phase DNA contents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barque J. P., Danon F., Peraudeau L., Yeni P., Larsen C. J. Characterization by human autoantibody of a nuclear antigen related to the cell cycle. EMBO J. 1983;2(5):743–749. doi: 10.1002/j.1460-2075.1983.tb01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K. G., Tanaka S., Hu S. Z., Wang T. S., Korn D. Intracellular localization of human DNA polymerase alpha with monoclonal antibodies. J Biol Chem. 1982 Jul 25;257(14):8391–8396. [PubMed] [Google Scholar]
- Black A., Freeman J. W., Zhou G. H., Busch H. Novel cell cycle-related nuclear proteins found in rat and human cells with monoclonal antibodies. Cancer Res. 1987 Jun 15;47(12):3266–3272. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Melamed M. R. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980 Sep;1(2):98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Galand P., Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989 Sep;22(5):383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Kurki P., Ogata K., Tan E. M. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods. 1988 Apr 22;109(1):49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- Landberg G., Tan E. M., Roos G. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res. 1990 Mar;187(1):111–118. doi: 10.1016/0014-4827(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Micko S., Craver J. L., McDivitt R. W. DNA flow cytometry of breast carcinoma after acetic-acid fixation. Cell Tissue Kinet. 1984 Mar;17(2):185–197. doi: 10.1111/j.1365-2184.1984.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Nakayasu H., Berezney R. Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol. 1989 Jan;108(1):1–11. doi: 10.1083/jcb.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. G., Cho K. R., Hsiang Y. H., Liu L. F., Coffey D. S. Growth-related elevations of DNA topoisomerase II levels found in Dunning R3327 rat prostatic adenocarcinomas. Cancer Res. 1987 Jun 15;47(12):3246–3250. [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Ritch P. S., Shackney S. E., Schuette W. H., Talbot T. L., Smith C. A. A practical graphical method for estimating the fraction of cells in S in DNA histograms from clinical tumor samples containing aneuploid cell populations. Cytometry. 1983 Jul;4(1):66–74. doi: 10.1002/cyto.990040110. [DOI] [PubMed] [Google Scholar]
- Robbins B. A., de la Vega D., Ogata K., Tan E. M., Nakamura R. M. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med. 1987 Sep;111(9):841–845. [PubMed] [Google Scholar]
- Shibui S., Hoshino T., Iwasaki K., Nomura K., Jastreboff M. M. Cell cycle phase dependent emergence of thymidylate synthase studied by monoclonal antibody (M-TS-4). Cell Tissue Kinet. 1989 May;22(3):259–268. doi: 10.1111/j.1365-2184.1989.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Shibui S., Hoshino T., Vanderlaan M., Gray J. W. Double labeling with iodo- and bromodeoxyuridine for cell kinetics studies. J Histochem Cytochem. 1989 Jul;37(7):1007–1011. doi: 10.1177/37.7.2659659. [DOI] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Mammalian DNA polymerases alpha and delta: current status in DNA replication. Biochemistry. 1988 Jun 28;27(13):4591–4595. doi: 10.1021/bi00413a001. [DOI] [PubMed] [Google Scholar]
- Taylor I. W., Hodson P. J., Green M. D., Sutherland R. L. Effects of tamoxifen on cell cycle progression of synchronous MCF-7 human mammary carcinoma cells. Cancer Res. 1983 Sep;43(9):4007–4010. [PubMed] [Google Scholar]
- Tubiana M., Pejovic M. H., Koscielny S., Chavaudra N., Malaise E. Growth rate, kinetics of tumor cell proliferation and long-term outcome in human breast cancer. Int J Cancer. 1989 Jul 15;44(1):17–22. doi: 10.1002/ijc.2910440104. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T., Tanaka S. Immunochemical detection of a primase activity related subunit of DNA polymerase alpha from human and mouse cells using the monoclonal antibody. Biochemistry. 1987 Dec 1;26(24):7749–7754. doi: 10.1021/bi00398a032. [DOI] [PubMed] [Google Scholar]
- van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Nuclear distribution of the Ki-67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res. 1989 Jun 1;49(11):2999–3006. [PubMed] [Google Scholar]
- van Dierendonck J. H., Keyzer R., van de Velde C. J., Cornelisse C. J. Subdivision of S-phase by analysis of nuclear 5-bromodeoxyuridine staining patterns. Cytometry. 1989 Mar;10(2):143–150. doi: 10.1002/cyto.990100205. [DOI] [PubMed] [Google Scholar]




