Abstract
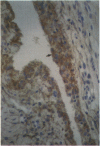
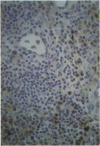
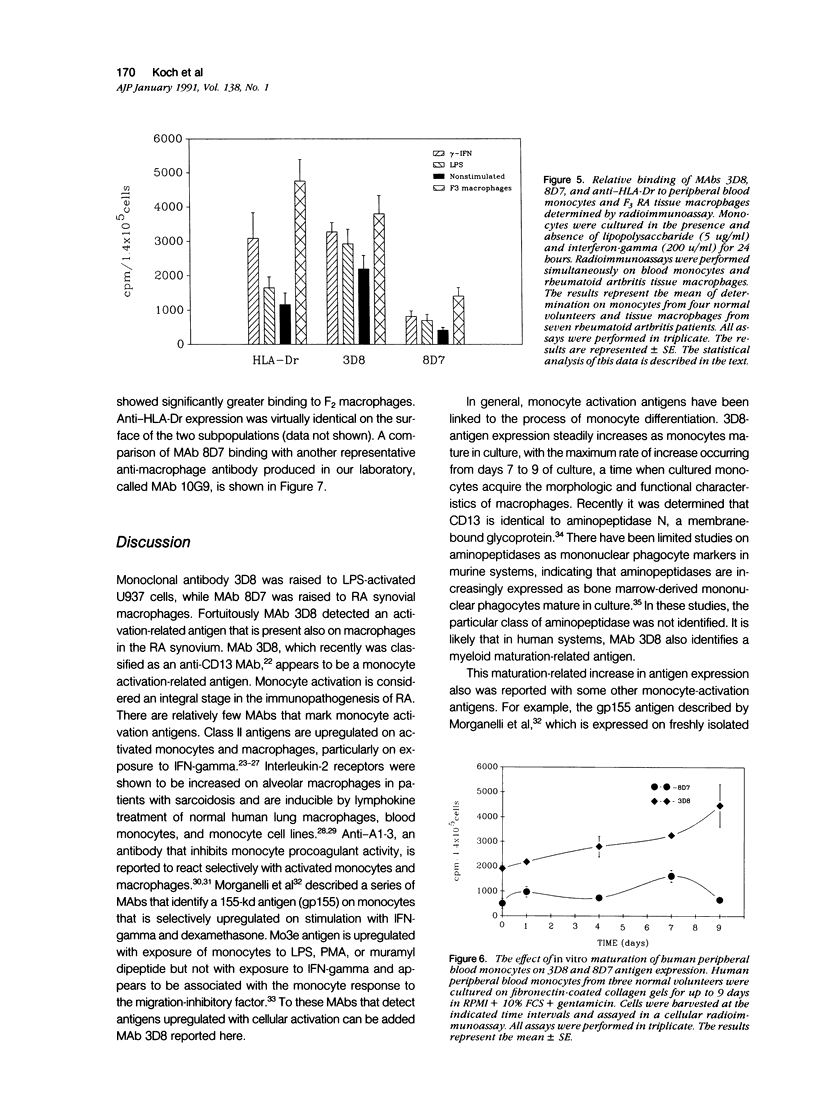
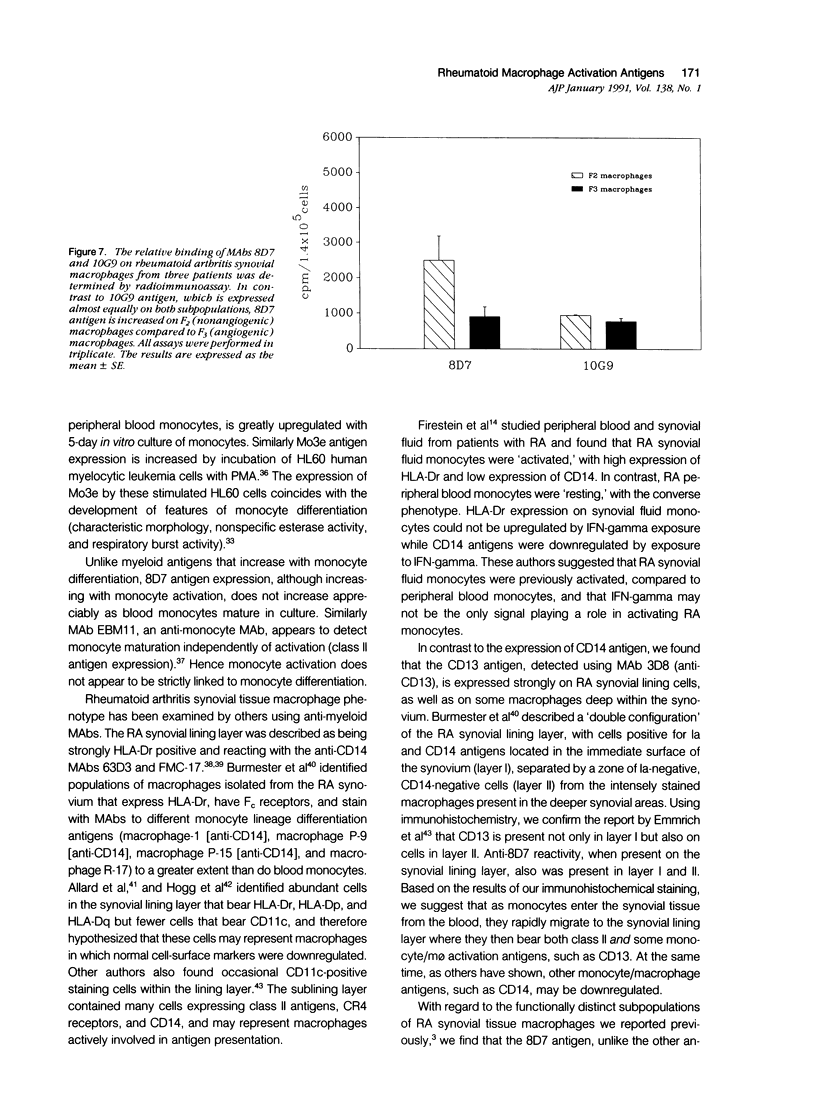
Monoclonal antibodies (MAbs) to functionally heterogeneous populations of human rheumatoid arthritis (RA) synovial tissue macrophages and lipopolysaccharide (LPS)-activated U937 cells were generated. These MAbs were used to characterize macrophages in situ in the synovial pannus and to study relative antigen expression on the surface of cells isolated from the synovium and from normal peripheral blood. Monoclonal antibody 3D8, an anti-CD13 MAb, reacts with an antigen expressed on the surface of blood monocytes and is a monocyte activation-related antigen that is upregulated by exposure of monocytes to interferon-gamma (IFN-gamma) and LPS. The expression of the 3D8 antigen increases in parallel with MHC class II antigen expression and also is upregulated in culture as monocytes mature to macrophages. 3D8 antigen is expressed strongly on RA synovial tissue lining cells, which are thought to be composed of macrophages. 8D7 antigen expression, detected by MAb 8D7, increases on blood monocytes on cellular activation with LPS and interferon-gamma, but in contrast to the 3D8 antigen, does not increase with monocyte maturation in vitro. The 8D7 antigen is expressed differentially on density-defined macrophage subpopulations isolated from RA synovial tissue and is expressed more strongly on macrophages that are nonangiogenic than those that are angiogenic.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Becker S. Interferons as modulators of human monocyte-macrophage differentiation. I. Interferon-gamma increases HLA-DR expression and inhibits phagocytosis of zymosan. J Immunol. 1984 Mar;132(3):1249–1254. [PubMed] [Google Scholar]
- Bielefeldt-Ohmann H., Sabara M., Lawman M. J., Griebel P., Babiuk L. A. A monoclonal antibody detects macrophage maturation antigen which appears independently of class II antigen expression. Reactivity of monoclonal EBM11 with bovine macrophages. J Immunol. 1988 Apr 1;140(7):2201–2209. [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Gebauer F., Weseloh G., Glückert K., Kinne R. Phenotype analysis of "inflammatory" macrophages in rheumatoid arthritis by two-colour cytofluorometry and immunohistology. Agents Actions. 1990 Jan;29(1-2):95–97. doi: 10.1007/BF01964729. [DOI] [PubMed] [Google Scholar]
- Ewan V. A., Cieplinski W., Hancock W. W., Goldschneider I., Boyd A. W., Rickles F. R. Production and characterization of a monoclonal antibody (A1-3) that binds selectively to activated monocytes and inhibits monocyte procoagulant activity. J Immunol. 1986 Apr 1;136(7):2408–2415. [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A. Hypothesis: in vitro evidence for the invasive and tumor-like properties of the rheumatoid pannus. J Rheumatol. 1983 Dec;10(6):845–851. [PubMed] [Google Scholar]
- Hancock W. W., Muller W. A., Cotran R. S. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J Immunol. 1987 Jan 1;138(1):185–191. [PubMed] [Google Scholar]
- Hancock W. W., Rickles F. R., Ewan V. A., Atkins R. C. Immunohistological studies with A1-3, a monoclonal antibody to activated human monocytes and macrophages. J Immunol. 1986 Apr 1;136(7):2416–2420. [PubMed] [Google Scholar]
- Harris E. D., Jr Recent insights into the pathogenesis of the proliferative lesion in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):68–72. doi: 10.1002/art.1780190111. [DOI] [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Levine H., Griffin J. D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med. 1985 Sep 1;162(3):1111–1116. doi: 10.1084/jem.162.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Holter W., Grunow R., Stockinger H., Knapp W. Recombinant interferon-gamma induces interleukin 2 receptors on human peripheral blood monocytes. J Immunol. 1986 Mar 15;136(6):2171–2175. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hu S. K., Mitcho Y. L., Oronsky A. L., Kerwar S. S. Studies on the effect of methotrexate on macrophage function. J Rheumatol. 1988 Feb;15(2):206–209. [PubMed] [Google Scholar]
- Iguchi T., Kurosaka M., Ziff M. Electron microscopic study of HLA-DR and monocyte/macrophage staining cells in the rheumatoid synovial membrane. Arthritis Rheum. 1986 May;29(5):600–613. doi: 10.1002/art.1780290504. [DOI] [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Schmid H., Weseloh G., Rohwer P., Kalden J. R. Changes in cell surface antigen expression on human articular chondrocytes induced by gamma-interferon. Induction of Ia antigens. Arthritis Rheum. 1987 Jan;30(1):64–74. doi: 10.1002/art.1780300109. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Functional heterogeneity of human rheumatoid synovial tissue macrophages. J Rheumatol. 1988 Jul;15(7):1058–1063. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986 Feb;39(2):233–238. doi: 10.1002/jlb.39.2.233. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Look A. T., Peiper S. C., Rebentisch M. B., Ashmun R. A., Roussel M. F., Lemons R. S., Le Beau M. M., Rubin C. M., Sherr C. J. Molecular cloning, expression, and chromosomal localization of the gene encoding a human myeloid membrane antigen (gp150). J Clin Invest. 1986 Oct;78(4):914–921. doi: 10.1172/JCI112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganelli P. M., Guyre P. M. IFN-gamma plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte-specific antigen. J Immunol. 1988 Apr 1;140(7):2296–2304. [PubMed] [Google Scholar]
- Nouri-Aria K. T., Williams R., Eddleston A. L. An enzyme immunoassay for the measurement of HLA-DR antigen expression. J Immunol Methods. 1988 Jun 28;111(1):83–88. doi: 10.1016/0022-1759(88)90062-2. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G., Seymour G. The involvement of interdigitating (antigen-presenting) cells in the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 1983 Feb;51(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Alvarez P. A., Brott D. A., Liu D. Y. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985 Dec;135(6):3869–3877. [PubMed] [Google Scholar]
- Wachsmuth E. D., Staber F. G. Changes in membrane-bound aminopeptidase on bone marrow-derived macrophages during their maturation in vitro. Exp Cell Res. 1977 Oct 15;109(2):269–276. doi: 10.1016/0014-4827(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985 Feb;134(2):982–989. [PubMed] [Google Scholar]