Abstract
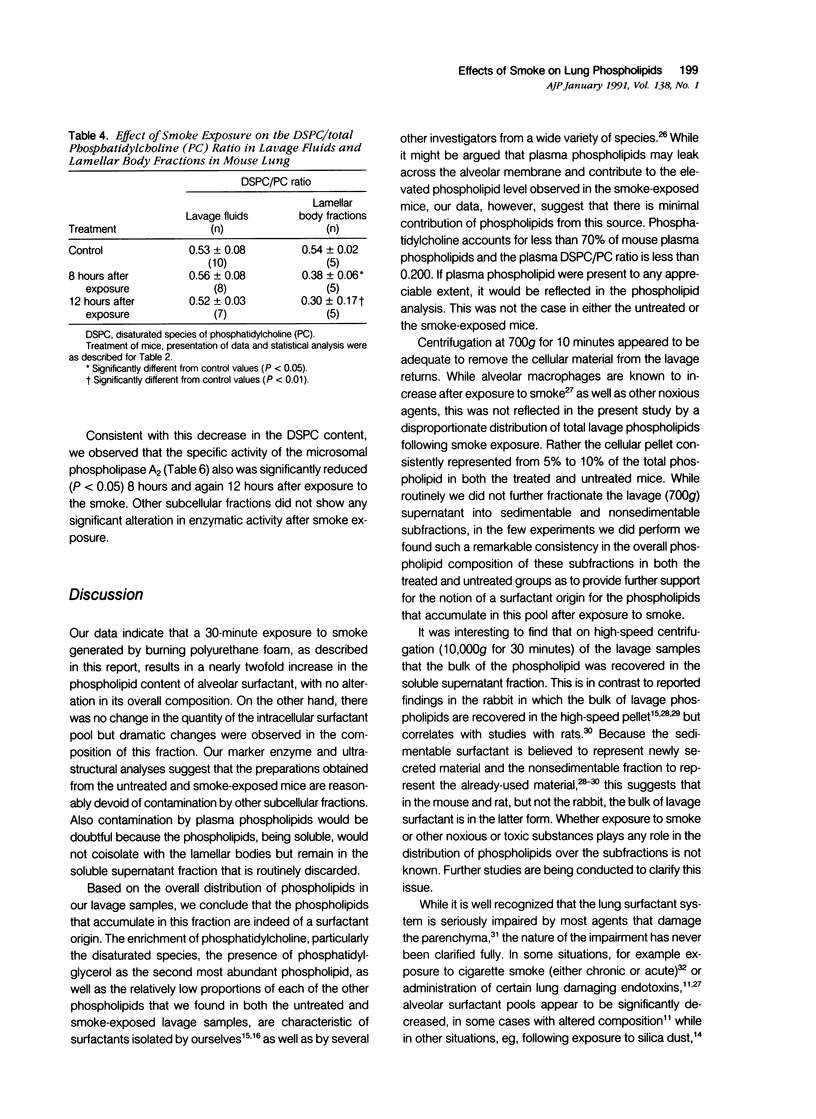
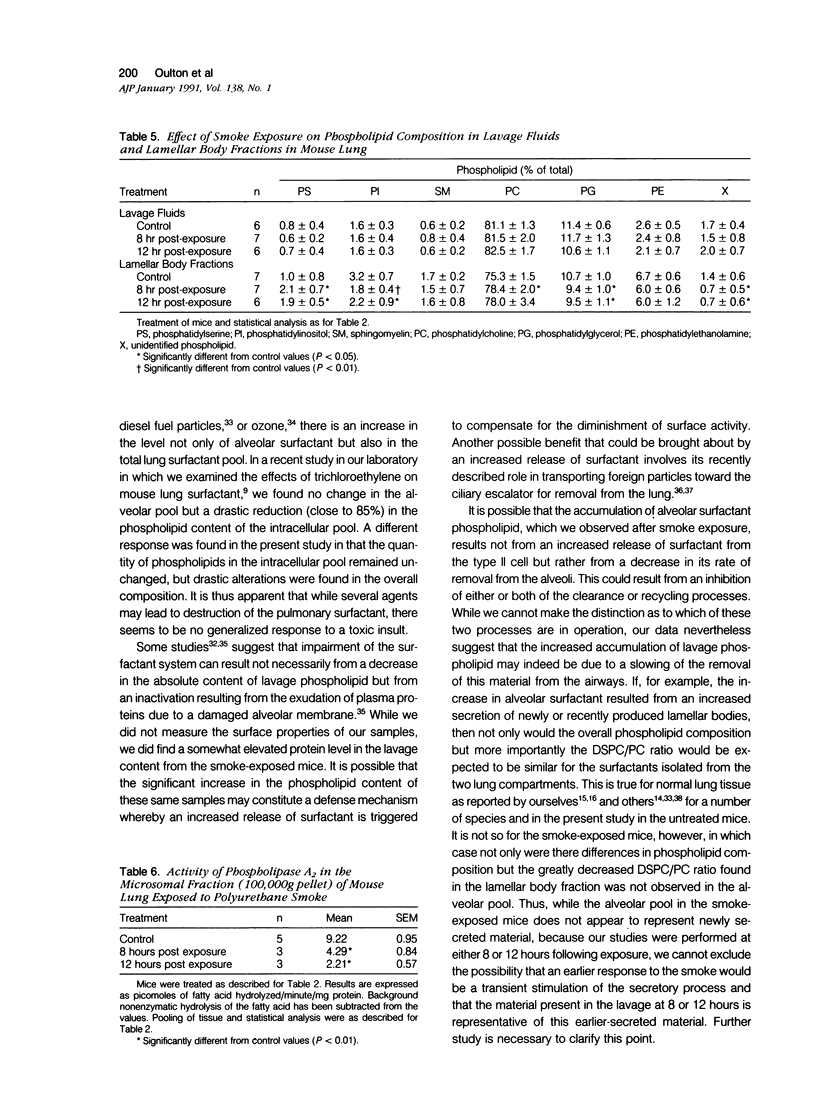
The effects of smoke inhalation on the pulmonary surfactant system were examined in mice exposed for 30 minutes to smoke generated from the burning of polyurethane foam. At 8 or 12 hours after exposure, surfactants were isolated separately from lung lavage (extracellular surfactant) and residual lung tissue (intracellular surfactant) for phospholipid analysis. Calcium-dependent phospholipase A2 (PLA2) was measured on a microsomal fraction prepared from the tissue homogenate. Smoke inhalation produced a twofold increase in extracellular surfactant total phospholipid. While there was no change in the total phospholipid or phosphatidylcholine (PC) content of the intracellular surfactant, smoke inhalation significantly decreased the disaturated species of PC (DSPC). The specific activity of PLA2 was reduced by more than 50% in both groups of exposed mice. Smoke inhalation appears to result in selective depletion of the DSPC of intracellular surfactant and PLA2 involved in its synthesis. This depletion may be compensated for by increased secretion or slower breakdown of the material present in the extracellular compartment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma K., Suzuki K. T. Effect of intratracheal instillation of cadmium chloride on phospholipids in alveolar wash fluid. Toxicology. 1987 Jun;44(3):321–328. doi: 10.1016/0300-483x(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Haller E. M., Shelley S. A., Montgomery M. R. Ozone-induced lamellar body responses in a rat model for alveolar injury and repair. Am J Pathol. 1988 Aug;132(2):330–344. [PMC free article] [PubMed] [Google Scholar]
- Baritussio A., Bellina L., Carraro R., Rossi A., Enzi G., Magoon M. W., Mussini I. Heterogeneity of alveolar surfactant in the rabbit: composition, morphology, and labelling of subfractions isolated by centrifugation of lung lavage. Eur J Clin Invest. 1984 Feb;14(1):24–29. doi: 10.1111/j.1365-2362.1984.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Bruni R., Baritussio A., Quaglino D., Gabelli C., Benevento M., Ronchetti I. P. Postnatal transformations of alveolar surfactant in the rabbit: changes in pool size, pool morphology and isoforms of the 32-38 kDa apolipoprotein. Biochim Biophys Acta. 1988 Feb 4;958(2):255–267. doi: 10.1016/0005-2760(88)90184-1. [DOI] [PubMed] [Google Scholar]
- Cohen M. A., Guzzardi L. J. Inhalation of products of combustion. Ann Emerg Med. 1983 Oct;12(10):628–632. doi: 10.1016/s0196-0644(83)80209-1. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Burns. N Engl J Med. 1985 Nov 28;313(22):1389–1398. doi: 10.1056/NEJM198511283132205. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Hook G. E. The relationship between intra- and extra-cellular surfactant phospholipids in the lungs of rabbits and the effects of silica-induced lung injury. Biochem J. 1986 Oct 1;239(1):59–67. doi: 10.1042/bj2390059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelson C. D., Chvapil M., Strom K. A., Vostal J. J. Pulmonary phospholipidosis in rats respiring air containing diesel particulates. Environ Res. 1987 Dec;44(2):260–271. doi: 10.1016/s0013-9351(87)80235-9. [DOI] [PubMed] [Google Scholar]
- Foster J. R., Cottrell R. C., Herod I. A., Atkinson H. A., Miller K. A comparative study of the pulmonary effects of NO2 in the rat and hamster. Br J Exp Pathol. 1985 Apr;66(2):193–204. [PMC free article] [PubMed] [Google Scholar]
- Giri S. N. Effects of intratracheal instillation of bleomycin on phospholipid synthesis in hamster lung tissue slices. Proc Soc Exp Biol Med. 1987 Dec;186(3):327–332. doi: 10.3181/00379727-186-42621. [DOI] [PubMed] [Google Scholar]
- Heimbach D. M., Waeckerle J. F. Inhalation injuries. Ann Emerg Med. 1988 Dec;17(12):1316–1320. doi: 10.1016/s0196-0644(88)80357-3. [DOI] [PubMed] [Google Scholar]
- Higenbottam T. Pulmonary surfactant and chronic lung disease. Eur J Respir Dis Suppl. 1987;153:222–228. [PubMed] [Google Scholar]
- Le Mesurier S. M., Lykke A. W., Stewart B. W. Reduced yield of pulmonary surfactant: patterns of response following administration of chemicals to rats by inhalation. Toxicol Lett. 1980 Jan;5(1):89–93. doi: 10.1016/0378-4274(80)90153-8. [DOI] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Miller K., Cottrell R. C. Adverse effects of toxins and drugs on the surfactant systems. Eur J Respir Dis Suppl. 1987;153:237–241. [PubMed] [Google Scholar]
- Nieman G. F., Clark W. R., Jr, Wax S. D., Webb S. R. The effect of smoke inhalation on pulmonary surfactant. Ann Surg. 1980 Feb;191(2):171–181. doi: 10.1097/00000658-198002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulton M., Dolphin M. Subcellular distribution of disaturated phosphatidylcholine in developing rabbit lung. Lipids. 1988 Jan;23(1):55–61. doi: 10.1007/BF02535305. [DOI] [PubMed] [Google Scholar]
- Oulton M., Fraser M., Dolphin M., Yoon R., Faulkner G. Quantification of surfactant pool sizes in rabbit lung during perinatal development. J Lipid Res. 1986 Jun;27(6):602–612. [PubMed] [Google Scholar]
- Oulton M., Martin T. R., Faulkner G. T., Stinson D., Johnson J. P. Developmental study of a lamellar body fraction isolated from human amniotic fluid. Pediatr Res. 1980 May;14(5):722–728. doi: 10.1203/00006450-198005000-00004. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Forkert P. G., Oulton M., Rasmusson M. G., Temple S., Fraser M. O., Whitefield S. Pulmonary toxicity of trichloroethylene: induction of changes in surfactant phospholipids and phospholipase A2 activity in the mouse lung. Exp Mol Pathol. 1988 Aug;49(1):141–150. doi: 10.1016/0014-4800(88)90028-7. [DOI] [PubMed] [Google Scholar]
- Spain C. L., Silbajoris R., Young S. L. Alterations of surfactant pools in fetal and newborn rat lungs. Pediatr Res. 1987 Jan;21(1):5–9. doi: 10.1203/00006450-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Tahvanainen J., Hallman M. Surfactant abnormality after endotoxin-induced lung injury in guinea-pigs. Eur J Respir Dis. 1987 Oct;71(4):250–258. [PubMed] [Google Scholar]
- Weiss J. M., Gebhardt K. F., Ziegler H., Rensch H. Rôle of surfactant in peripheral transport mechanisms. Eur J Respir Dis Suppl. 1987;153:205–208. [PubMed] [Google Scholar]



