Abstract
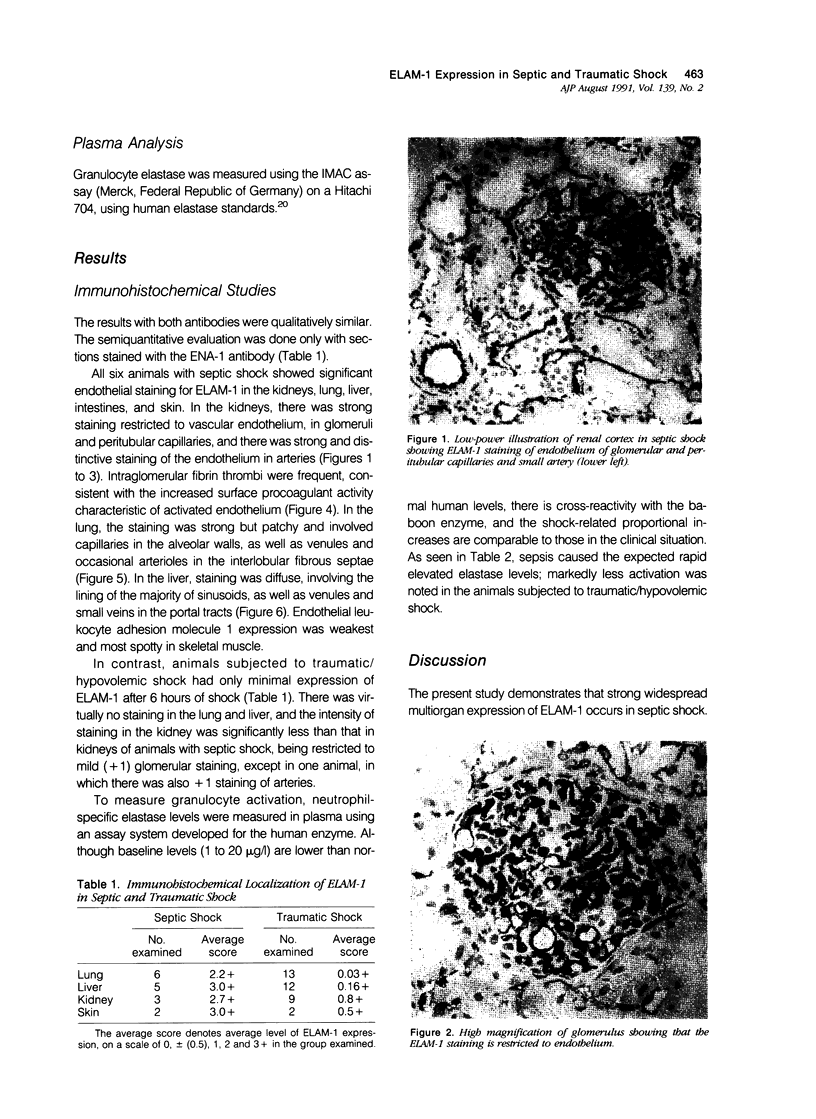
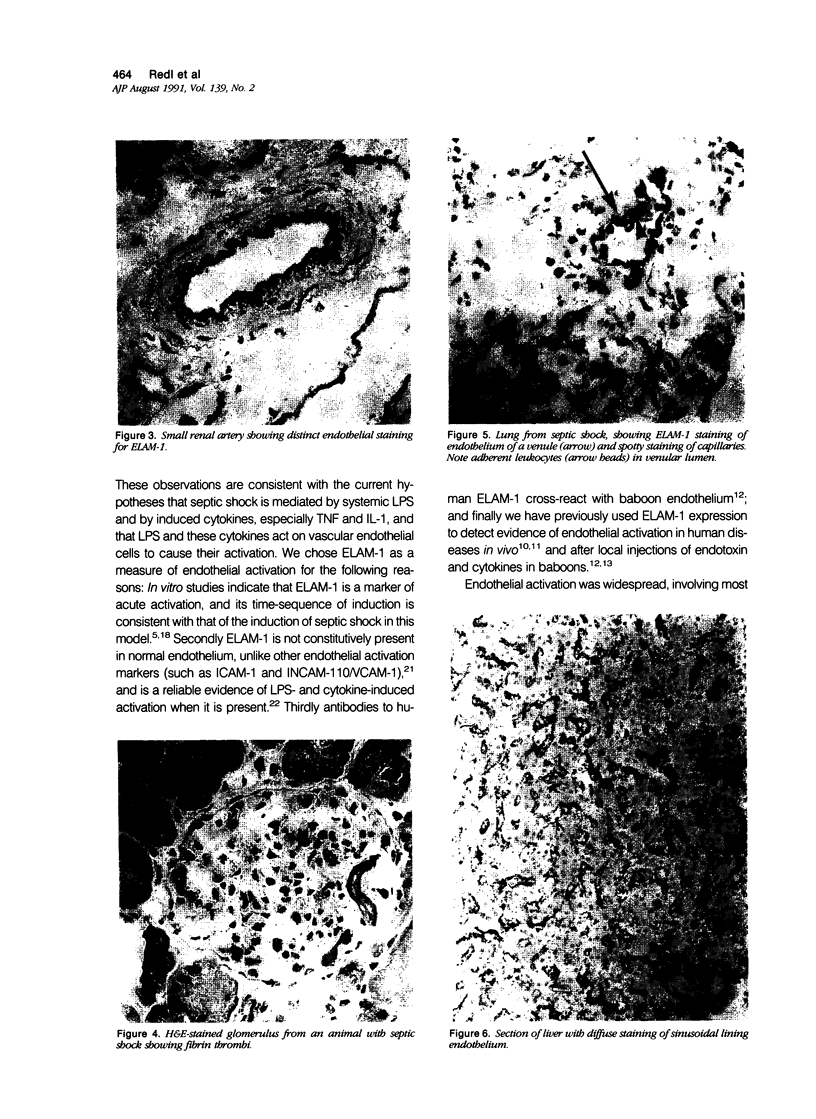
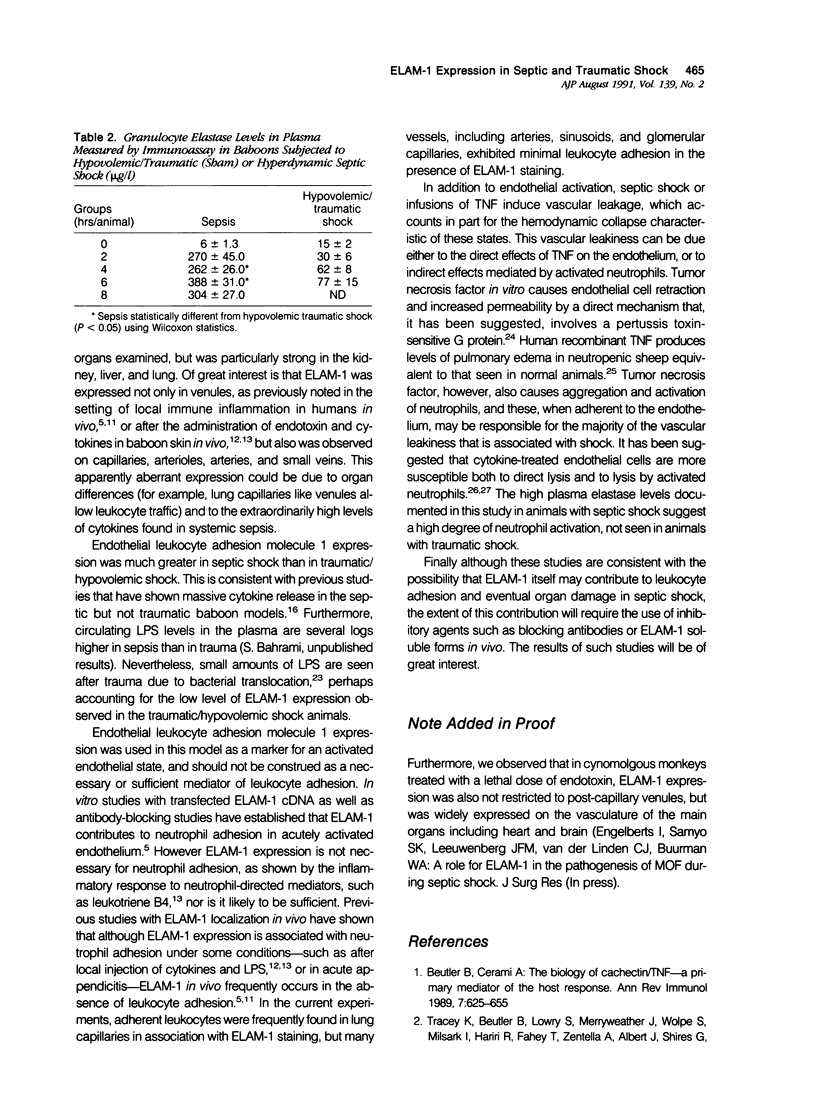
Baboons were subjected to septic or traumatic/hypovolemic shock and their tissues were examined for the de novo expression of endothelial leukocyte adhesion molecule 1 (ELAM-1), using immunohistochemical techniques. In animals with septic shock induced with live Escherichia coli, there was widespread expression of ELAM-1, recognized by monoclonal antibodies H4/18 or ENA-1 in most tissues examined with strong staining in the lung, liver, and kidneys. Endothelial leukocyte adhesion molecule 1 expression was evident in capillaries, venules, small veins, arterioles, and arteries. In contrast, baboons with traumatic/hypovolemic shock had minimal levels of focal ELAM expression in all organs studied. Similarly evidence of neutrophil activation, measured by granulocyte elastase levels in the plasma was much more pronounced in animals with septic shock. The study documents that lipopolysaccharide (LPS)- and cytokine-induced endothelial activation occurs in vivo in septic shock. Much higher levels of ELAM-1 expression and plasma granulocyte-elastase titer in septic shock, as contrasted with traumatic/hypovolemic shock, are consistent with the higher levels of circulating tumor necrosis factor, other cytokines, and LPS in sepsis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Brett J., Gerlach H., Nawroth P., Steinberg S., Godman G., Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989 Jun 1;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Pober J. S. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J Am Soc Nephrol. 1990 Sep;1(3):225–235. doi: 10.1681/ASN.V13225. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Pober J. S. Effects of cytokines on vascular endothelium: their role in vascular and immune injury. Kidney Int. 1989 Apr;35(4):969–975. doi: 10.1038/ki.1989.80. [DOI] [PubMed] [Google Scholar]
- Horvath C. J., Ferro T. J., Jesmok G., Malik A. B. Recombinant tumor necrosis factor increases pulmonary vascular permeability independent of neutrophils. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9219–9223. doi: 10.1073/pnas.85.23.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Lang H., Jochum M., Fritz H., Redl H. Validity of the elastase assay in intensive care medicine. Prog Clin Biol Res. 1989;308:701–706. [PubMed] [Google Scholar]
- Leeuwenberg J. F., Jeunhomme T. M., Buurman W. A. Induction of an activation antigen on human endothelial cells in vitro. Eur J Immunol. 1989 Apr;19(4):715–720. doi: 10.1002/eji.1830190422. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Recruitment of neutrophils in the local endotoxin response: association with de novo endothelial expression of endothelial leukocyte adhesion molecule-1. Lab Invest. 1991 Feb;64(2):295–299. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pretorius J. P., Schlag G., Redl H., Botha W. S., Goosen D. J., Bosman H., van Eeden A. F. The 'lung in shock' as a result of hypovolemic-traumatic shock in baboons. J Trauma. 1987 Dec;27(12):1344–1353. doi: 10.1097/00005373-198712000-00005. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Varani J., Bendelow M. J., Sealey D. E., Kunkel S. L., Gannon D. E., Ryan U. S., Ward P. A. Tumor necrosis factor enhances susceptibility of vascular endothelial cells to neutrophil-mediated killing. Lab Invest. 1988 Aug;59(2):292–295. [PubMed] [Google Scholar]
- Ward P. A., Varani J. Mechanisms of neutrophil-mediated killing of endothelial cells. J Leukoc Biol. 1990 Jul;48(1):97–102. doi: 10.1002/jlb.48.1.97. [DOI] [PubMed] [Google Scholar]








