Abstract
AKR mice develop hyperplasia of the thymus before the development of retrovirus-associated lymphoma at that site. This hyperplasia, first detectable in AKR/J mice by 4 weeks of age and in AKR/C mice by 4 to 5 months of age, is characterized by an enlarged thymic medulla that contains T and B lymphocytes. In contrast to the general population of thymocytes, most of these T and B lymphocytes have a mature immunophenotype that includes expression of high levels of the MEL-14-defined (gp90) 'homing receptor' for peripheral lymph node high endothelial venules. In vivo homing studies reveal a marked increase in traffic of peripheral lymphocytes (T more than B) to the hyperplastic thymuses of old AKR mice as compared to histologically normal thymuses of age-matched BALB/c and C57BL/Ka mice or young AKR mice. These changes correlate chronologically with changes in retrovirus antigen expression in AKR thymuses and suggest a role for the traffic of lymphocytes from the periphery to the thymus in response to local antigenic stimulation in the pathogenesis of thymic hyperplasia in AKR mice.
Full text
PDF

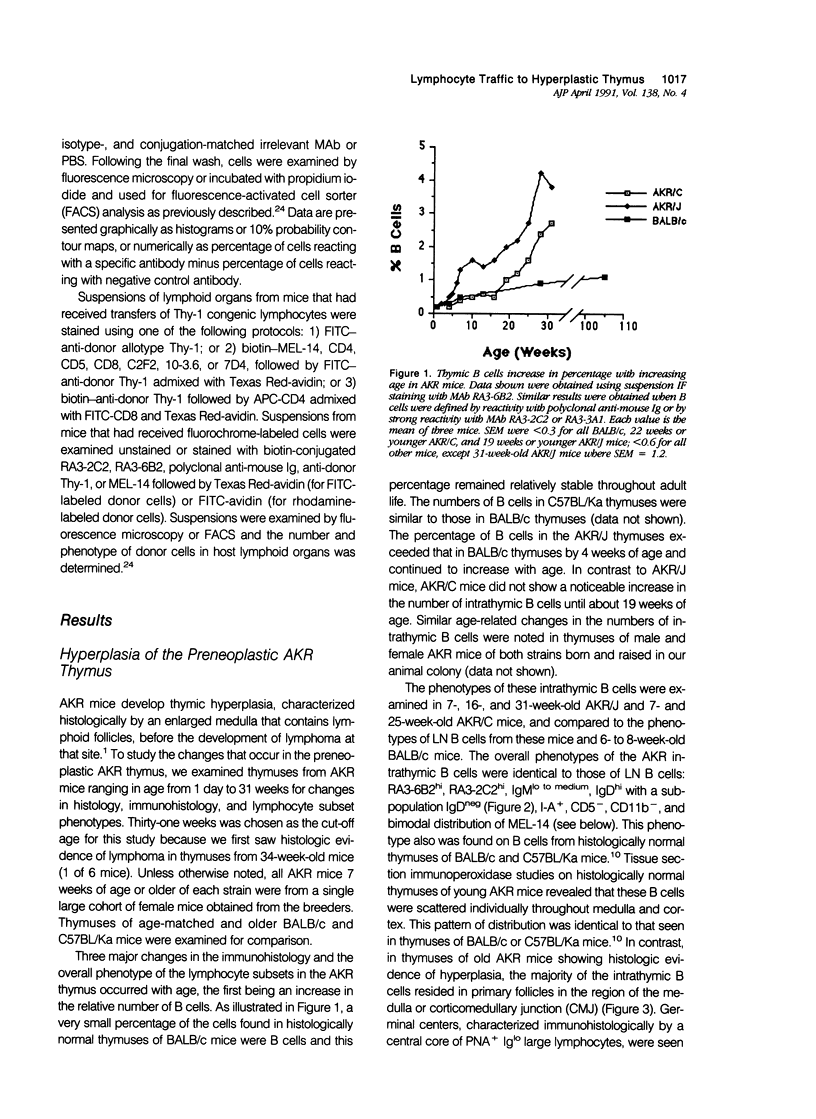
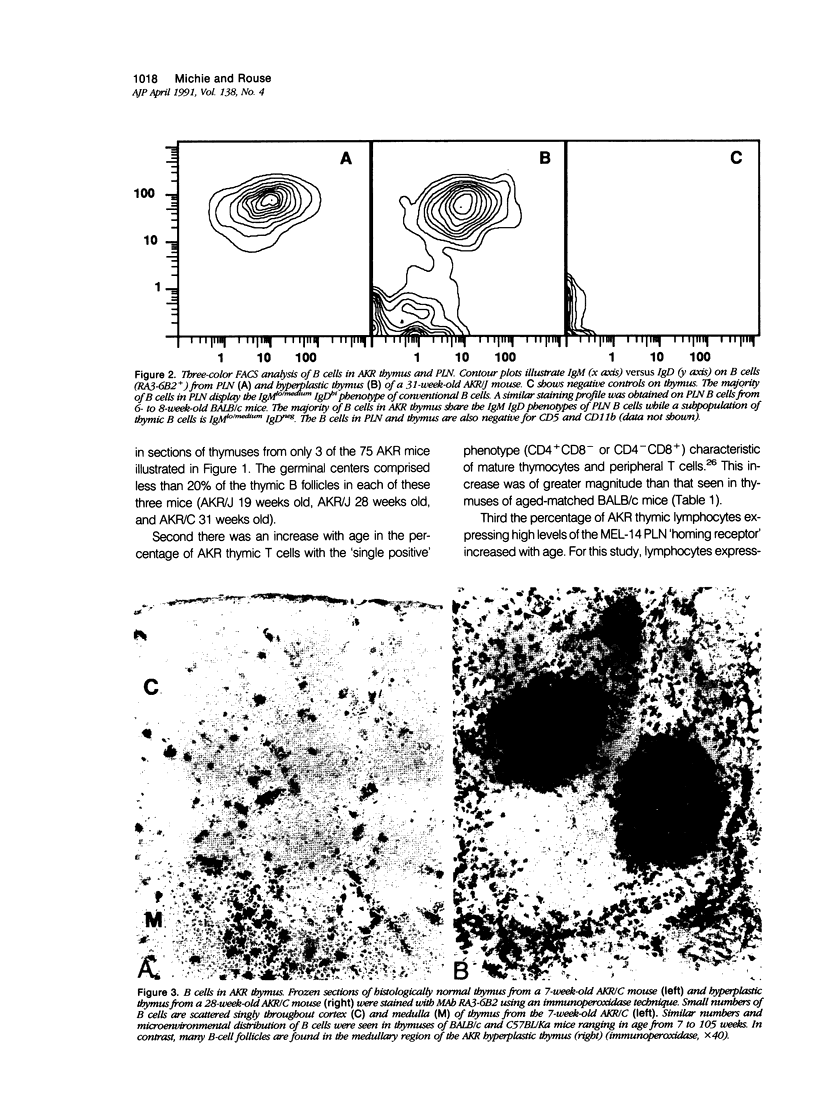
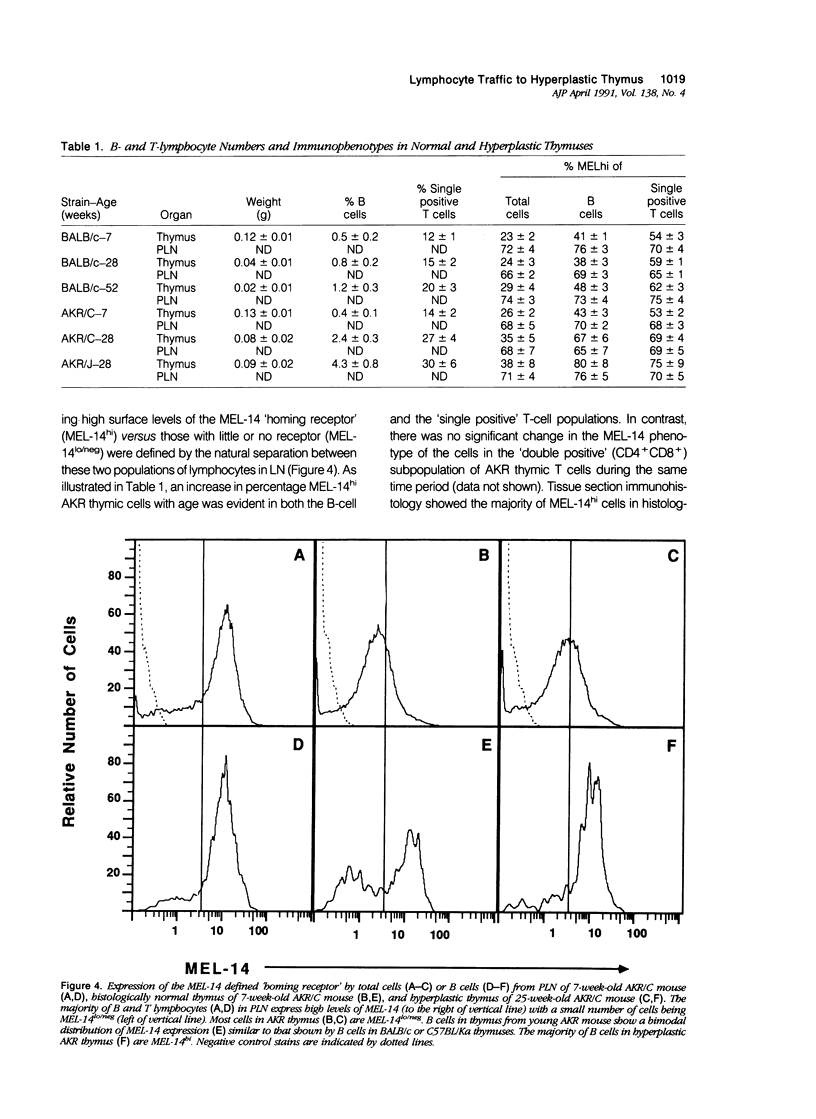
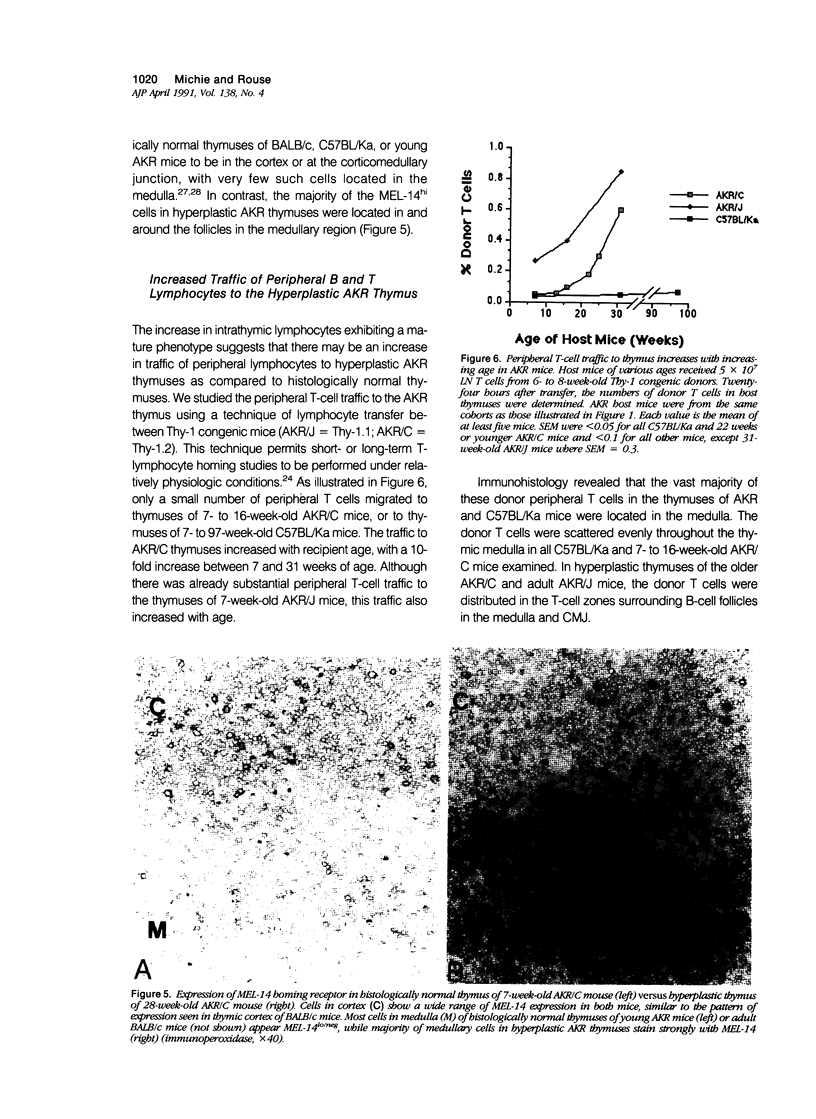
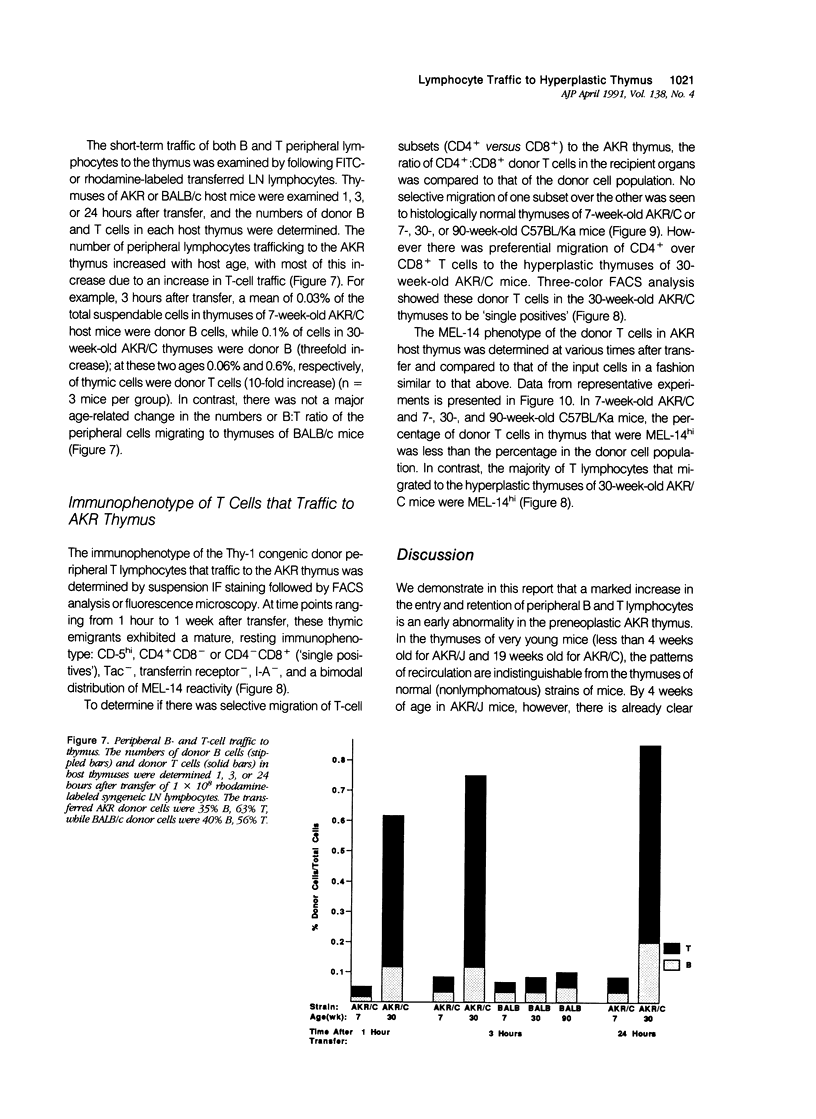
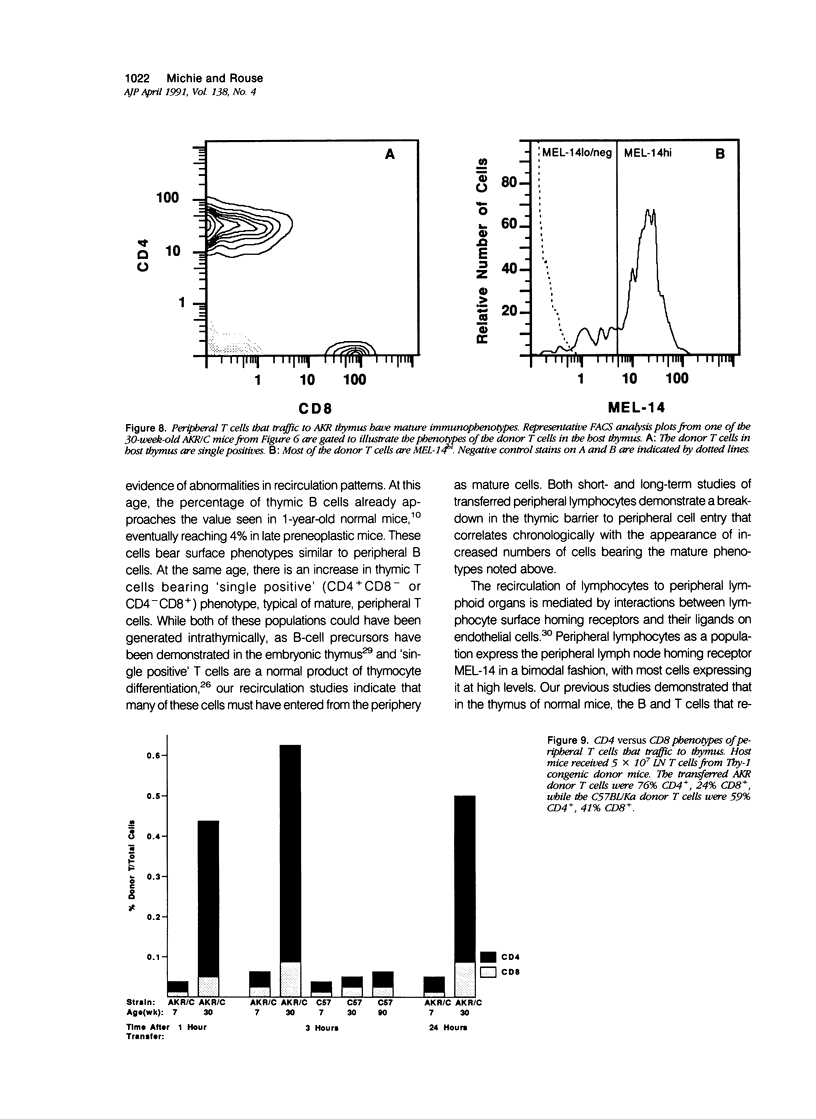
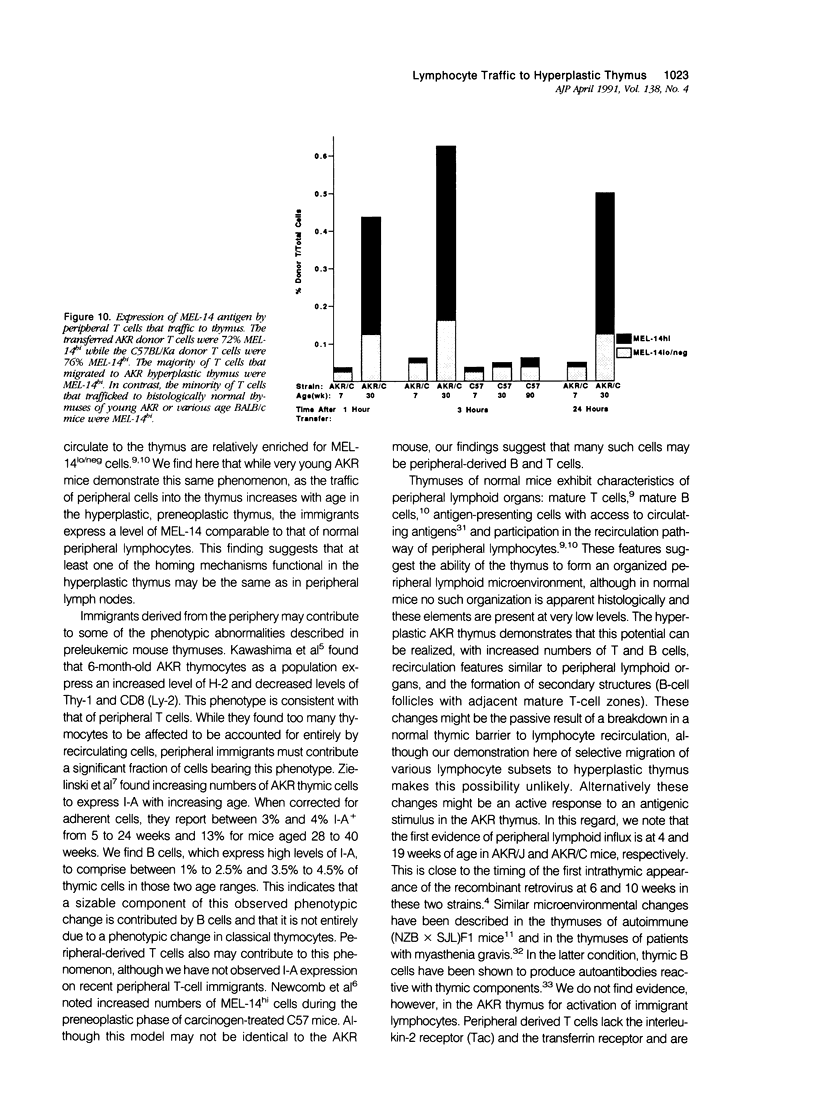


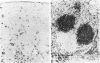
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. II. Potential application to studies of lymphocyte migration and maturation. J Immunol Methods. 1980;37(2):109–121. doi: 10.1016/0022-1759(80)90196-9. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Barrois R., Jacobson E. B. Migration of peripheral T and B cells into the thymus of aging (NZB X SJL)F1 female mice. Cell Immunol. 1984 Feb;83(2):292–301. doi: 10.1016/0008-8749(84)90308-3. [DOI] [PubMed] [Google Scholar]
- Evans L. H. Characterization of polytropic MuLVs from three-week-old AKR/J mice. Virology. 1986 Aug;153(1):122–136. doi: 10.1016/0042-6822(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Malik F. G. Class II polytropic murine leukemia viruses (MuLVs) of AKR/J mice: possible role in the generation of class I oncogenic polytropic MuLVs. J Virol. 1987 Jun;61(6):1882–1892. doi: 10.1128/jvi.61.6.1882-1892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Stockert E., Takahashi T., Old L. J. Age-related changes in cell surface antigens of preleukemic AKR thymocytes. J Exp Med. 1976 Jul 1;144(1):193–208. doi: 10.1084/jem.144.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Thorson J. A., McAlmont T. H., Horowitz M., Cowdery J. S., Ballas Z. K. Role of the transferrin receptor in lymphocyte growth: a rat IgG monoclonal antibody against the murine transferrin receptor produces highly selective inhibition of T and B cell activation protocols. J Immunol. 1987 Apr 15;138(8):2422–2426. [PubMed] [Google Scholar]
- Kimoto H., Shirasawa T., Taniguchi M., Takemori T. B cell precursors are present in the thymus during early development. Eur J Immunol. 1989 Jan;19(1):97–104. doi: 10.1002/eji.1830190116. [DOI] [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Kyewski B. A., Fathman C. G., Rouse R. V. Intrathymic presentation of circulating non-MHC antigens by medullary dendritic cells. An antigen-dependent microenvironment for T cell differentiation. J Exp Med. 1986 Feb 1;163(2):231–246. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Horak I., Ihle J. N. Mechanisms in T cell leukemogenesis. II. T cell responses of preleukemic BALB/c mice to Moloney leukemia virus antigens. J Immunol. 1981 Feb;126(2):715–722. [PubMed] [Google Scholar]
- McGrath M. S., Pillemer E., Weissman I. L. Murine leukaemogenesis: monoclonal antibodies to T-cell determinants arrest T-lymphoma cell proliferation. Nature. 1980 May 22;285(5762):259–261. doi: 10.1038/285259a0. [DOI] [PubMed] [Google Scholar]
- Michie S. A., Kirkpatrick E. A., Rouse R. V. Rare peripheral T cells migrate to and persist in normal mouse thymus. J Exp Med. 1988 Nov 1;168(5):1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S. A., Rouse R. V. Study of murine T cell migration using the Thy-1 allotypic marker. Demonstration of antigen-specific homing to lymph node germinal centers. Transplantation. 1988 Jul;46(1):98–104. doi: 10.1097/00007890-198807000-00018. [DOI] [PubMed] [Google Scholar]
- Newcomb E. W., Pellicer A., Cordon C. Comparative analysis and anatomic distribution of ras p21, IL-2R, and MEL-14 in malignant and hyperplastic murine thymus. Am J Pathol. 1990 Feb;136(2):307–317. [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Reichert R. A., Gallatin W. M., Butcher E. C., Weissman I. L. A homing receptor-bearing cortical thymocyte subset: implications for thymus cell migration and the nature of cortisone-resistant thymocytes. Cell. 1984 Aug;38(1):89–99. doi: 10.1016/0092-8674(84)90529-4. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., Reichert R. A., Gallatin W. M., Weissman I. L., Butcher E. C. Localization of lymphocyte subpopulations in peripheral lymphoid organs: directed lymphocyte migration and segregation into specific microenvironments. Am J Anat. 1984 Jul;170(3):391–405. doi: 10.1002/aja.1001700313. [DOI] [PubMed] [Google Scholar]
- Shortman K., Wilson A., Van Ewijk W., Scollay R. Phenotype and localization of thymocytes expressing the homing receptor-associated antigen MEL-14: arguments for the view that most mature thymocytes are located in the medulla. J Immunol. 1987 Jan 15;138(2):342–351. [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Willcox H. N., Newsom-Davis J. Immunohistological studies of the thymus in myasthenia gravis. Correlation with clinical state and thymocyte culture responses. J Neuroimmunol. 1982 Dec;3(4):319–335. doi: 10.1016/0165-5728(82)90035-2. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Willcox H. N., Newsom-Davis J., Calder L. R. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol. 1983 Nov;54(2):378–386. [PMC free article] [PubMed] [Google Scholar]
- Zielinski C. C., Datta S. K., Waksal S. D. Department of Medicine, Tufts University School of Medicine, Boston, Massachusetts. Immunogenetics. 1981;14(1-2):169–176. doi: 10.1007/BF00344310. [DOI] [PubMed] [Google Scholar]